Acids and Bases. General propertiesPage
3
3
The amino acid glycine - amphoteric
Itís an acid and a base!
Loss of H+
Gain of H+
H2N-CH2-COOH
H3N+-CH2-COOH
H2N-CH2-COO-
Chime structure
Slide 21
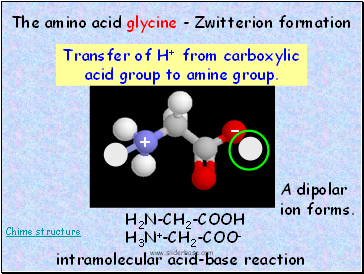
The amino acid glycine - Zwitterion formation
Transfer of H+ from carboxylic acid group to amine group.
H2N-CH2-COOH
H3N+-CH2-COO-
+
-
A dipolar ion forms.
intramolecular acid-base reaction
Chime structure
Slide 22
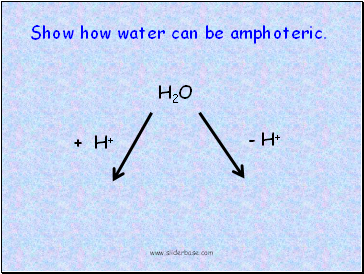
Show how water can be amphoteric.
H2O
Slide 23
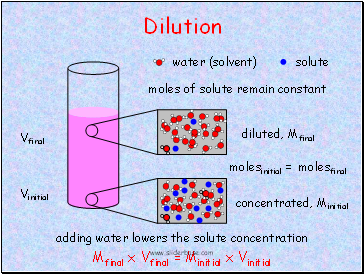
Dilution
water (solvent)
solute
concentrated, Minitial
diluted, Mfinal
adding water lowers the solute concentration
moles of solute remain constant
Vinitial
Vfinal
molesinitial = molesfinal
Mfinal x Vfinal = Minitial x Vinitial
Slide 24
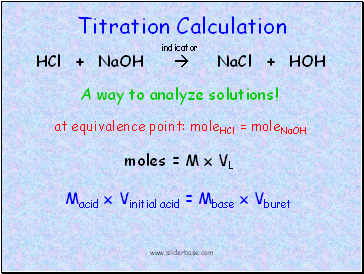
Titration Calculation
HCl + NaOH NaCl + HOH
at equivalence point: moleHCl = moleNaOH
moles = M x VL
Macid x Vinitial acid = Mbase x Vburet
A way to analyze solutions!
indicator
Contents
- General properties
- Definitions
- The Bronsted-Lowry Concept
- Neutralization
- Strong and Weak Acids/Bases
- What is acid rain?
- Dilution
- Titration Calculation
Last added presentations
- The Effects of Radiation on Living Things
- Health Physics
- Sound
- Waves & Sound
- Thermal Energy
- Newton's laws of motion
- Radiation Safety and Operations