Acids and Bases. General propertiesPage
2
2
HA H+ + A-
At any one time, only a fraction of the molecules are dissociated.
Slide 12
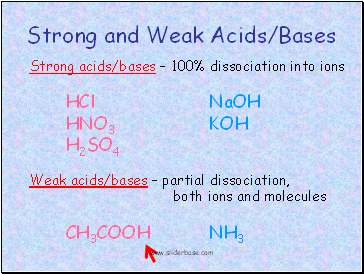
Strong and Weak Acids/Bases
Strong acids/bases – 100% dissociation into ions
HCl NaOH
HNO3 KOH
H2SO4
Weak acids/bases – partial dissociation, both ions and molecules
CH3COOH NH3
Slide 13
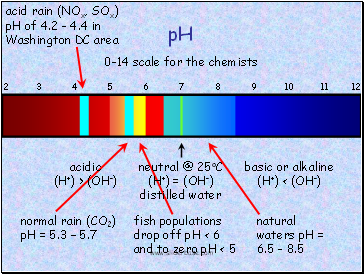
pH
2
3
4
5
6
7
8
9
10
11
12
neutral @ 25oC
(H+) = (OH-)
distilled water
acidic
(H+) > (OH-)
basic or alkaline
(H+) < (OH-)
natural waters pH = 6.5 - 8.5
normal rain (CO2)
pH = 5.3 – 5.7
acid rain (NOx, SOx)
pH of 4.2 - 4.4 in Washington DC area
0-14 scale for the chemists
fish populations
drop off pH < 6 and to zero pH < 5
Slide 14
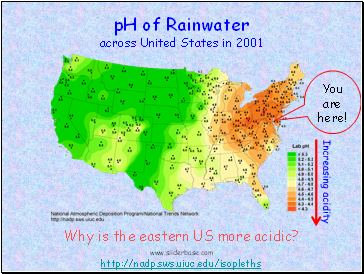
You are here!
http://nadp.sws.uiuc.edu/isopleths
pH of Rainwater
across United States in 2001
Why is the eastern US more acidic?
air masses
Slide 15
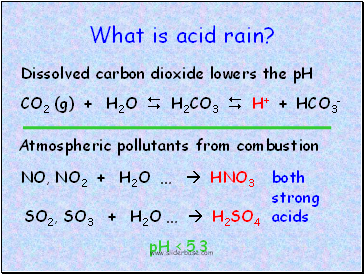
What is acid rain?
CO2 (g) + H2O H2CO3 H+ + HCO3-
Dissolved carbon dioxide lowers the pH
Atmospheric pollutants from combustion
NO, NO2 + H2O … HNO3
SO2, SO3 + H2O … H2SO4
both
strong
acids
pH < 5.3
Slide 16
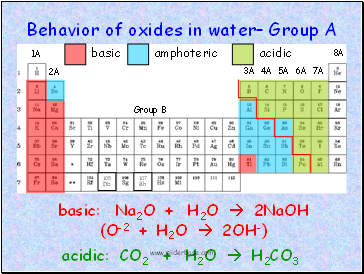
Behavior of oxides in water– Group A
basic
amphoteric
acidic
3A 4A 5A 6A 7A
1A
2A
8A
Group B
basic: Na2O + H2O 2NaOH
(O-2 + H2O 2OH-)
acidic: CO2 + H2O H2CO3
Slide 17
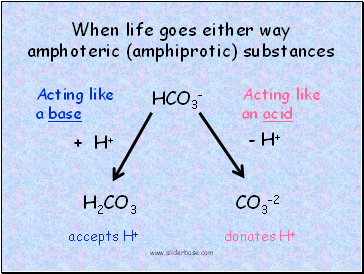
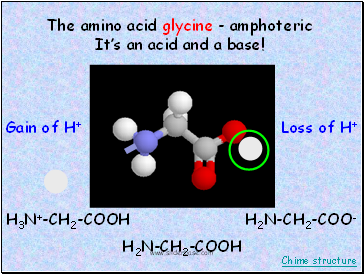
When life goes either way
amphoteric (amphiprotic) substances
HCO3-
H2CO3
CO3-2
Acting like
a base
Acting like an acid
accepts H+
donates H+
Slide 18
pH
1
2
3
4
5
6
8
9
10
11
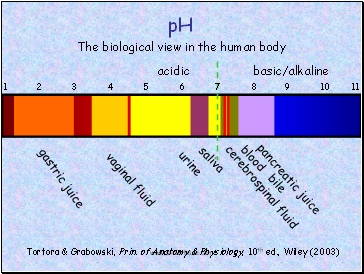
The biological view in the human body
gastric juice
vaginal fluid
urine
saliva
cerebrospinal fluid
blood
pancreatic juice
bile
acidic
basic/alkaline
7
Tortora & Grabowski, Prin. of Anatomy & Physiology, 10th ed., Wiley (2003)
Slide 19
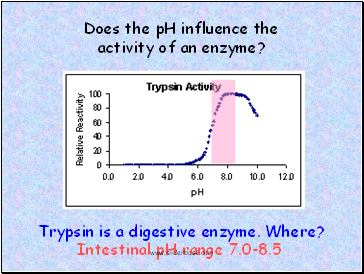
Does the pH influence the activity of an enzyme?
Trypsin is a digestive enzyme. Where?
Intestinal pH range 7.0-8.5
Slide 20
Contents
- General properties
- Definitions
- The Bronsted-Lowry Concept
- Neutralization
- Strong and Weak Acids/Bases
- What is acid rain?
- Dilution
- Titration Calculation
Last added presentations
- Solar Thermal Energy
- Gravitation
- Radiation
- Solar Energy
- History of Modern Astronomy
- Newton’s Law of Gravity
- Motion