Atoms and their structurePage
3
3
+
+
+
+
+
+
+
-
-
-
-
-
-
-
Some drops would hover
Slide 25
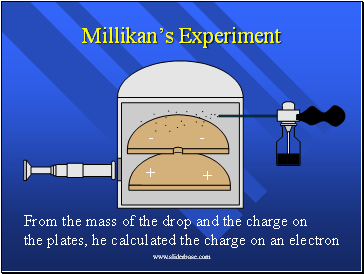
Millikan’s Experiment
From the mass of the drop and the charge on
the plates, he calculated the charge on an electron
+
+
-
-
Slide 26
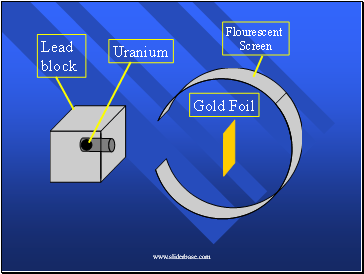
Rutherford’s Experiment
Ernest Rutherford English physicist. (1910)
Believed the plum pudding model of the atom was correct.
Wanted to see how big they are.
Used radioactivity.
Alpha particles - positively charged pieces given off by uranium.
Shot them at gold foil which can be made a few atoms thick.
Slide 27
Rutherford’s experiment
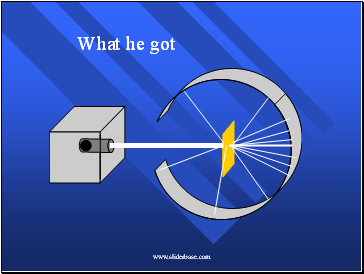
When the alpha particles hit a florescent screen, it glows.
Here’s what it looked like (pg 72)
Slide 28
Lead block
Uranium
Gold Foil
Flourescent
Screen
Slide 29
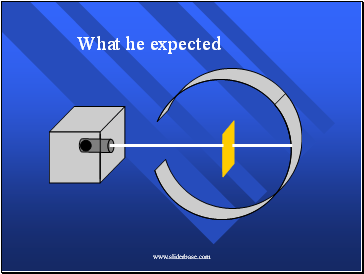
He Expected
The alpha particles to pass through without changing direction very much.
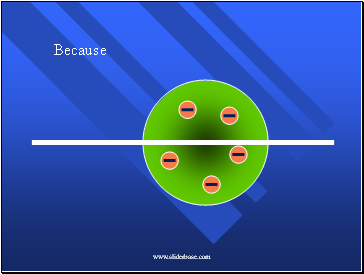
Because…
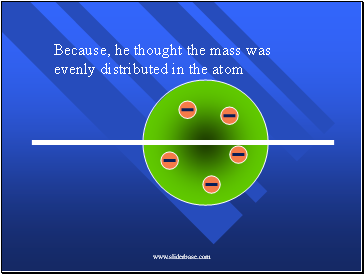
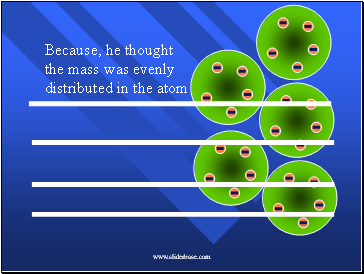
The positive charges were spread out evenly. Alone they were not enough to stop the alpha particles.
Slide 30
What he expected
Slide 31
Because
Slide 32
Because, he thought the mass was evenly distributed in the atom
Slide 33
Because, he thought the mass was evenly distributed in the atom
Slide 34
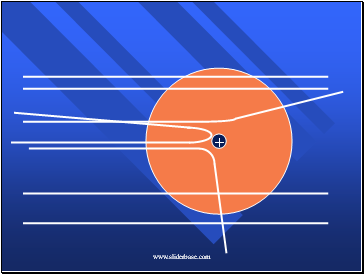
What he got
Slide 35
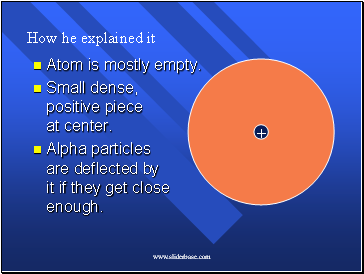
How he explained it
Atom is mostly empty.
Small dense, positive piece at center.
Alpha particles are deflected by it if they get close enough.
Slide 36
Slide 37
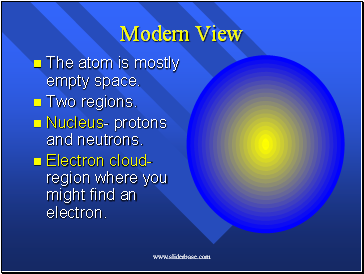
Modern View
The atom is mostly empty space.
Two regions.
Nucleus- protons and neutrons.
Electron cloud- region where you might find an electron.
Slide 38
Density and the Atom
Contents
- History of the atom
- Another Greek
- Who Was Right?
- Who’s Next?
- Dalton’s Atomic Theory
- Parts of Atoms
- Thomsom’s Model
- Rutherford’s Experiment
- He Expected
- Modern View
- Density and the Atom
- Structure of the Atom
- Size of an atom
- Counting the Pieces
- Isotopes
- Naming Isotopes
- Atomic Mass
- Calculating averages
Last added presentations
- Waves & Sound
- Newton’s Law of Gravity
- Ch 9 Nuclear Radiation
- Radiation
- Motion
- The Effects of Radiation on Living Things
- Radioactivity and Nuclear Reactions