Bonding, Molecular Shape & StructurePage
2
2
Total Number of valence electrons = 5 + (3 x 7) = 26
Total Number of valence electrons = 4 + (2 x 6) = 16
Slide 10
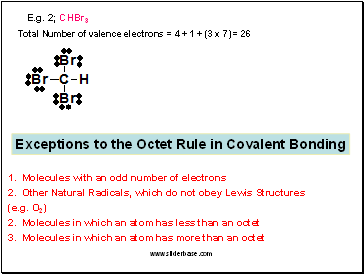
E.g. 2; CHBr3
Total Number of valence electrons = 4 + 1 + (3 x 7) = 26
Exceptions to the Octet Rule in Covalent Bonding
Molecules with an odd number of electrons
Other Natural Radicals, which do not obey Lewis Structures
(e.g. O2)
Molecules in which an atom has less than an octet
3. Molecules in which an atom has more than an octet
Slide 11
1. Odd Number of Electrons
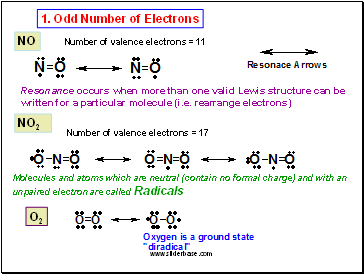
NO
Number of valence electrons = 11
NO2
Number of valence electrons = 17
O2
Resonance occurs when more than one valid Lewis structure can be written for a particular molecule (i.e. rearrange electrons)
Molecules and atoms which are neutral (contain no formal charge) and with an unpaired electron are called Radicals
Slide 12
2. Less than an Octet
Includes Lewis acids such as halides of B, Al and compounds of Be
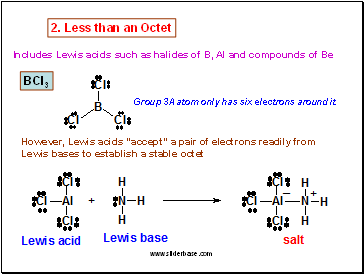
BCl3
Group 3A atom only has six electrons around it
However, Lewis acids “accept” a pair of electrons readily from Lewis bases to establish a stable octet
Slide 13
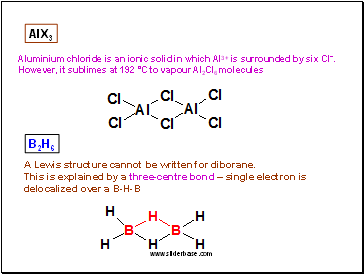
AlX3
Aluminium chloride is an ionic solid in which Al3+ is surrounded by six Cl-.
However, it sublimes at 192 °C to vapour Al2Cl6 molecules
B2H6
A Lewis structure cannot be written for diborane.
This is explained by a three-centre bond – single electron is delocalized over a B-H-B
Slide 14
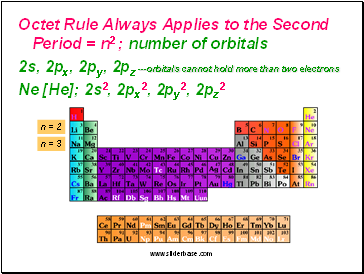
Octet Rule Always Applies to the Second Period = n2 ; number of orbitals
2s, 2px, 2py, 2pz ---orbitals cannot hold more than two electrons
Ne [He]; 2s2, 2px2, 2py2, 2pz2
n = 2
n = 3
Slide 15
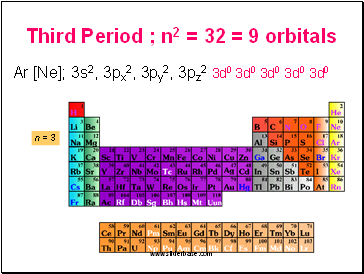
Third Period ; n2 = 32 = 9 orbitals
Ar [Ne]; 3s2, 3px2, 3py2, 3pz2 3d0 3d0 3d0 3d0 3d0
n = 3
Slide 16
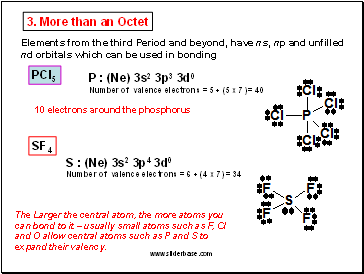
3. More than an Octet
PCl5
Elements from the third Period and beyond, have ns, np and unfilled nd orbitals which can be used in bonding
P : (Ne) 3s2 3p3 3d0
Number of valence electrons = 5 + (5 x 7) = 40
10 electrons around the phosphorus
SF4
S : (Ne) 3s2 3p4 3d0
Number of valence electrons = 6 + (4 x 7) = 34
The Larger the central atom, the more atoms you can bond to it – usually small atoms such as F, Cl and O allow central atoms such as P and S to expand their valency.
Slide 17
Contents
- Lewis Symbols
- Pauling scale of electronegativity;
- Electronegativity is dictated by
- Shapes of Molecules
- Trigonal Pyramidal
- Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
- Valence Shell Electron-Pair Repulsion Theory (VSEPR)
- Molecules with Expanded Valence Shells
- Hydrogen Bonding & Water
- Dipole-dipole Attractive Forces
Last added presentations
- Solar Thermal Energy
- Newton's Laws
- Resource Acquisition and Transport in Vascular Plants
- Gravitation
- Space Radiation
- Magnetic field uses sound waves to ignite sun's ring of fire
- Direct heat utilization of geothermal energy