Bonding, Molecular Shape & StructurePage
6
6
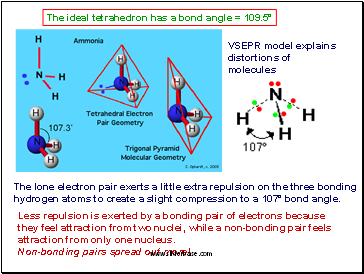
The ideal tetrahedron has a bond angle = 109.5°
The lone electron pair exerts a little extra repulsion on the three bonding hydrogen atoms to create a slight compression to a 107° bond angle.
VSEPR model explains distortions of molecules
Less repulsion is exerted by a bonding pair of electrons because they feel attraction from two nuclei, while a non-bonding pair feels attraction from only one nucleus.
Non-bonding pairs spread out more!
Slide 35
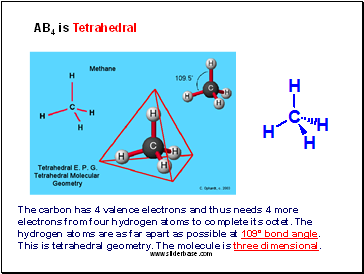
AB4 is Tetrahedral
The carbon has 4 valence electrons and thus needs 4 more electrons from four hydrogen atoms to complete its octet. The hydrogen atoms are as far apart as possible at 109° bond angle. This is tetrahedral geometry. The molecule is three dimensional.
Slide 36
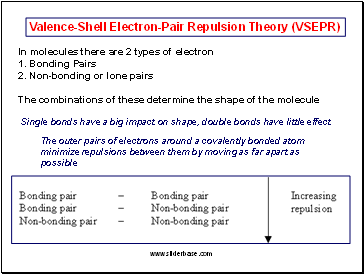
Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
In molecules there are 2 types of electron
1. Bonding Pairs
2. Non-bonding or lone pairs
The combinations of these determine the shape of the molecule
Single bonds have a big impact on shape, double bonds have little effect
The outer pairs of electrons around a covalently bonded atom minimize repulsions between them by moving as far apart as possible
Slide 37
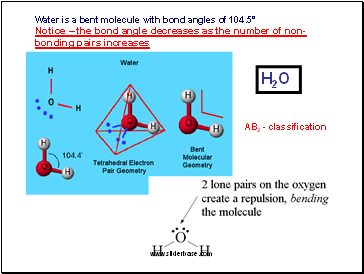
Water is a bent molecule with bond angles of 104.5°
Notice – the bond angle decreases as the number of non-bonding pairs increases
AB2 - classification
H2O
Slide 38
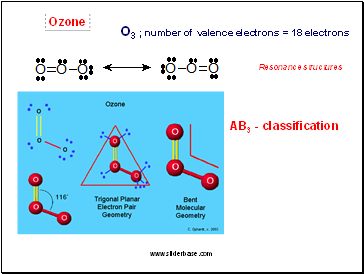
Ozone
O3 ; number of valence electrons = 18 electrons
Resonance structures
AB3 - classification
Slide 39
Valence Shell Electron-Pair Repulsion Theory (VSEPR)
Procedure
Sum the total Number of Valence Electrons
Drawing the Lewis Structure
2. The atom usually written first in the chemical formula is the Central atom in the Lewis structure
Complete the octet bonded to the Central atom. However, elements in the third row have empty d-orbitals which can be used for bonding.
If there are not enough electrons to give the central atom an octet try multiple bonds.
Predicting the Shape of the Molecule
Sum the Number of Electron Domains around the Central Atom in the Lewis Structure; Single = Double = Triple Bonds = Non-Bonding Lone Pair of Electrons = One Electron Domain
From the Total Number of Electron Domains, Predict the Geometry and Bond Angle(s); 2 (Linear = 180º); 3 (Trigonal Planar = 120º); 4 (Tetrahedral = 109.5º); 5 (Trigonal Bipyramidal = 120º and 90º); 6 (Octahedral = 90º)
Contents
- Lewis Symbols
- Pauling scale of electronegativity;
- Electronegativity is dictated by
- Shapes of Molecules
- Trigonal Pyramidal
- Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
- Valence Shell Electron-Pair Repulsion Theory (VSEPR)
- Molecules with Expanded Valence Shells
- Hydrogen Bonding & Water
- Dipole-dipole Attractive Forces
Last added presentations
- Upcoming Classes
- Newton’s laws of motion
- Motion
- Sound
- Madame Marie Curie
- Radiation Safety and Operations
- Direct heat utilization of geothermal energy