Bonding, Molecular Shape & StructurePage
9
9
The ease with which an external electric field can induce a dipole (alter the electron distribution) with a molecule is referred to as the "polarizability" of that molecule
The greater the polarizability of a molecule the easier it is to induce a momentary dipole and the stronger the dispersion forces
Larger molecules tend to have greater polarizability
Their electrons are further away from the nucleus (any asymmetric distribution produces a larger dipole due to larger charge separation)
The number of electrons is greater (higher probability of asymmetric distribution)
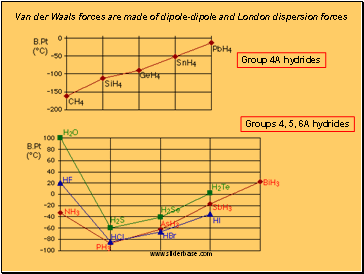
thus, dispersion forces tend to increase with increasing molecular mass
Dispersion forces are also present between polar/non-polar and polar/polar molecules (i.e. between all molecules)
Slide 54
Group 4A hydrides
Groups 4, 5, 6A hydrides
Van der Waals forces are made of dipole-dipole and London dispersion forces
Contents
- Lewis Symbols
- Pauling scale of electronegativity;
- Electronegativity is dictated by
- Shapes of Molecules
- Trigonal Pyramidal
- Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
- Valence Shell Electron-Pair Repulsion Theory (VSEPR)
- Molecules with Expanded Valence Shells
- Hydrogen Bonding & Water
- Dipole-dipole Attractive Forces
Last added presentations
- Upcoming Classes
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Newton’s Laws of Motion
- Motion
- Newton's Laws
- Madame Marie Curie