The Building Blocks of Matter AtomsPage
3
3
What do you notice about the number
of quarks in the neutron and proton?
Slide 15
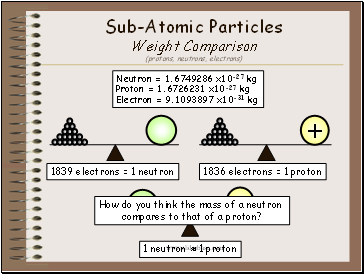
Sub-Atomic Particles Weight Comparison (protons, neutrons, electrons)
Neutron = 1.6749286 x10-27 kg Proton = 1.6726231 x10-27 kg Electron = 9.1093897 x10-31 kg
1836 electrons = 1 proton
1839 electrons = 1 neutron
How do you think the mass of a neutron
compares to that of a proton?
1 neutron ≈ 1 proton
Slide 16
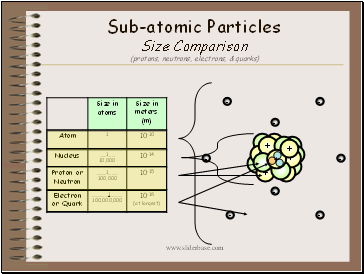
Sub-atomic Particles Size Comparison (protons, neutrons, electrons, & quarks)
+
+
+
+
-
-
-
-
-
-
-
-
+
Slide 17
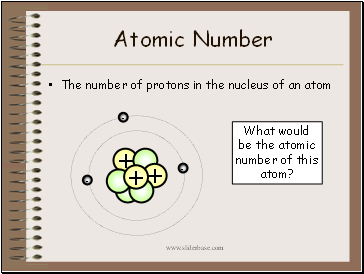
Atomic Number
The number of protons in the nucleus of an atom
-
-
-
What would be the atomic number of this atom?
Slide 18
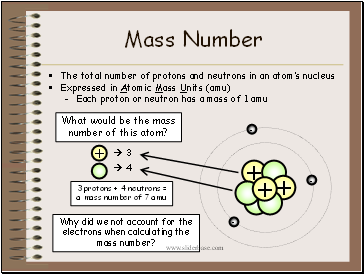
Mass Number
The total number of protons and neutrons in an atom’s nucleus
Expressed in Atomic Mass Units (amu)
Each proton or neutron has a mass of 1 amu
-
-
-
What would be the mass number of this atom?
+
3
4
3 protons + 4 neutrons = a mass number of 7 amu
Why did we not account for the electrons when calculating the mass number?
Slide 19
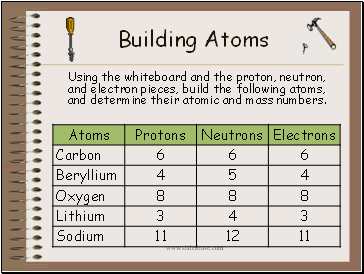
Building Atoms
Using the whiteboard and the proton, neutron, and electron pieces, build the following atoms, and determine their atomic and mass numbers.
Slide 20
Atom Builder
Using the interactive website link below, practice building atoms.
http://www.pbs.org/wgbh/aso/tryit/atom/
Using the classzone.com link below, click on the “Build an Atom” simulation and practice building atoms.
http://www.classzone.com/books/ml_sci_physical/page_build.cfm?id=resour_ch1&u=2##
Slide 21
FORCES IN THE ATOM
Gravitational Force
Electromagnetic Force
Strong Force
Weak Force
Slide 22
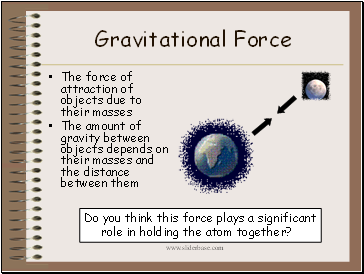
Gravitational Force
The force of attraction of objects due to their masses
The amount of gravity between objects depends on their masses and the distance between them
Do you think this force plays a significant
role in holding the atom together?
Slide 23
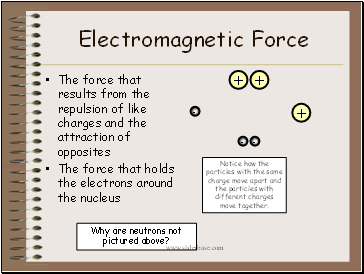
Electromagnetic Force
The force that results from the repulsion of like charges and the attraction of opposites
Contents
- Matter
- Atoms
- Atoms are so small that…
- Let’s Experiment
- Results
- Protons (+)
- Neutrons
- Electrons (-)
- Hydrogen (H) Atom
- Oxygen (O) Atom
- Sodium (Na) Atom
- The Atom’s “Center”
- Quarks
- Atomic Number
- Mass Number
- Building Atoms
- Atom Builder
- Gravitational Force
- Electromagnetic Force
- Strong Force
- Weak Force
- Isotopes
- Atomic Mass
- Ion
- Building Ions
Last added presentations
- Space Radiation
- Direct heat utilization of geothermal energy
- Radiation Safety and Operations
- Soil and Plant Nutrition
- Geophysical Concepts, Applications and Limitations
- Newton's laws of motion
- Understanding Heat Transfer, Conduction, Convection and Radiation