The Building Blocks of Matter AtomsPage
4
4
The force that holds the electrons around the nucleus
-
+
+
+
-
-
Notice how the particles with the same charge move apart and the particles with different charges move together.
Why are neutrons not pictured above?
Slide 24
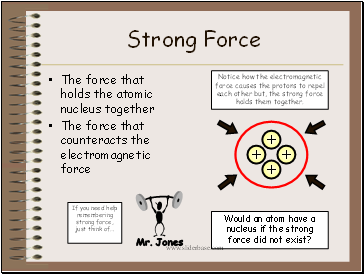
Strong Force
The force that holds the atomic nucleus together
The force that counteracts the electromagnetic force
Mr. Jones
If you need help remembering strong force, just think of…
+
+
+
+
Notice how the electromagnetic force causes the protons to repel each other but, the strong force holds them together.
Would an atom have a nucleus if the strong force did not exist?
Slide 25
-
n
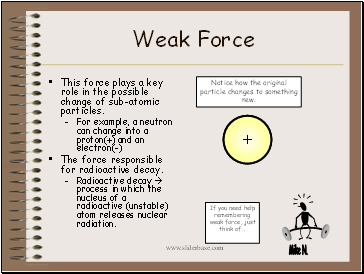
Weak Force
This force plays a key role in the possible change of sub-atomic particles.
For example, a neutron can change into a proton(+) and an electron(-)
The force responsible for radioactive decay.
Radioactive decay process in which the nucleus of a radioactive (unstable) atom releases nuclear radiation.
Mike N.
+
If you need help remembering weak force, just think of…
Notice how the original particle changes to something new.
Slide 26
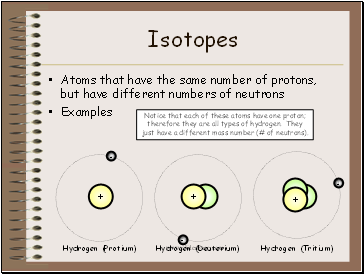
Isotopes
Atoms that have the same number of protons, but have different numbers of neutrons
Examples
+
-
+
-
+
-
Hydrogen (Protium)
Hydrogen (Deuterium)
Hydrogen (Tritium)
Notice that each of these atoms have one proton; therefore they are all types of hydrogen. They just have a different mass number (# of neutrons).
Slide 27
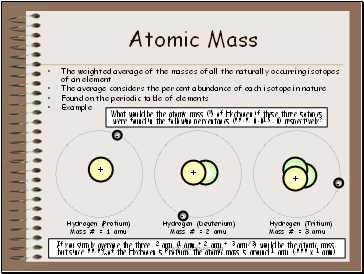
Atomic Mass
The weighted average of the masses of all the naturally occurring isotopes of an element
The average considers the percent abundance of each isotope in nature
Found on the periodic table of elements
Example
+
-
+
-
+
-
Hydrogen (Protium)
Mass # = 1 amu
Hydrogen (Deuterium)
Mass # = 2 amu
Hydrogen (Tritium)
Mass # = 3 amu
If you simply average the three, 2 amu (1 amu + 2 amu + 3 amu/3) would be the atomic mass, but since 99.9% of the Hydrogen is Protium, the atomic mass is around 1 amu (.999 x 1 amu)
What would be the atomic mass (≈) of Hydrogen if these three isotopes
were found in the following percentages (99.9, 0.015, 0) respectively?
Slide 28
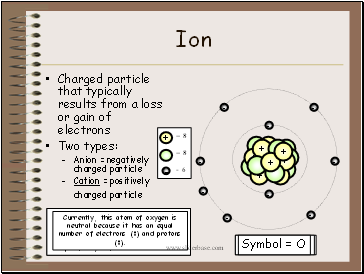
Ion
Symbol = O2+
Charged particle that typically results from a loss or gain of electrons
Two types:
Anion = negatively charged particle
Cation = positively charged particle
Contents
- Matter
- Atoms
- Atoms are so small that…
- Let’s Experiment
- Results
- Protons (+)
- Neutrons
- Electrons (-)
- Hydrogen (H) Atom
- Oxygen (O) Atom
- Sodium (Na) Atom
- The Atom’s “Center”
- Quarks
- Atomic Number
- Mass Number
- Building Atoms
- Atom Builder
- Gravitational Force
- Electromagnetic Force
- Strong Force
- Weak Force
- Isotopes
- Atomic Mass
- Ion
- Building Ions
Last added presentations
- Newton’s Law of Gravity
- Space Radiation
- Sensory and Motor Mechanisms
- Ch 9 Nuclear Radiation
- Buoyancy
- Radioactivity and Nuclear Reactions
- Thermal Energy