The Building Blocks of Matter AtomsPage
5
5
+
+
+
+
-
-
-
-
-
-
-
-
+
-
Now that this atom of oxygen just gained an electron, it is no longer neutral or an atom. It is now considered an ion (anion). This ion has more electrons (9) than protons (8).
9
6
Symbol = O1-
Now that three electrons were lost, the number of electrons (6) and protons (8) is still unbalanced; therefore, it is still an ion, but now it is specifically referred to as a cation.
Currently, this atom of oxygen is neutral because it has an equal number of electrons (8) and protons (8).
Symbol = O
Slide 29
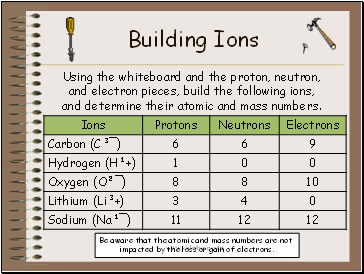
Building Ions
Using the whiteboard and the proton, neutron, and electron pieces, build the following ions, and determine their atomic and mass numbers.
Be aware that the atomic and mass numbers are not
impacted by the loss or gain of electrons.
Contents
- Matter
- Atoms
- Atoms are so small that…
- Let’s Experiment
- Results
- Protons (+)
- Neutrons
- Electrons (-)
- Hydrogen (H) Atom
- Oxygen (O) Atom
- Sodium (Na) Atom
- The Atom’s “Center”
- Quarks
- Atomic Number
- Mass Number
- Building Atoms
- Atom Builder
- Gravitational Force
- Electromagnetic Force
- Strong Force
- Weak Force
- Isotopes
- Atomic Mass
- Ion
- Building Ions
Last added presentations
- Health Physics
- Newton’s laws of motion
- Newton’s Law of Gravity
- Newton's laws of motion
- Heat-Energy on the Move
- Sensory and Motor Mechanisms
- History of Modern Astronomy
© 2010-2026 powerpoint presentations