Solar SystemPage
1
1
Slide 1
Announcements
Homework 7 due today
Pick up Homework 8
Next test will be next week
Slide 2
Our Solar System and Others
16 October 2006
Slide 3
Today:
Solar system patterns
Age of the solar system (and a crash course in nuclear physics)
Formation of the solar system
Planets around other stars
Slide 4
Solar System Patterns
The solar system is very flat. Why?
Nearly all the planets orbit and spin in the same direction. Why?
Inner planets are small; outer planets are big. Why?
Inner planets are mostly solid; outer planets are mostly gas and liquid. Why?
Inner planets have little hydrogen and helium; outer planets have lots. Why?
Partial answers are not hard to guess…
Detailed answers require an account of how the solar system formed.
Slide 5
How old is the solar system?
Layered rocks imply an age of at least millions of years.
Earth’s hot interior implies an upper limit on its age (as does sun’s energy output).
Age of the earth?
William Thomson, Lord Kelvin
Slide 6
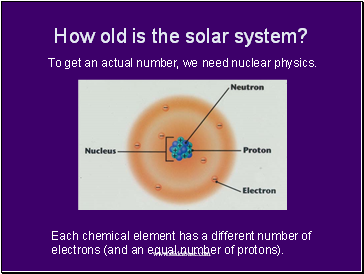
How old is the solar system?
To get an actual number, we need nuclear physics.
Each chemical element has a different number of electrons (and an equal number of protons).
Slide 7
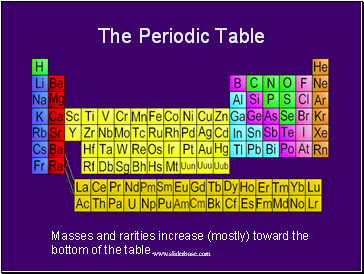
The Periodic Table
Masses and rarities increase (mostly) toward the bottom of the table.
Slide 8
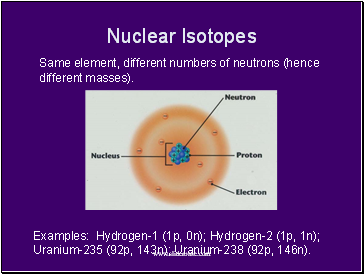
Nuclear Isotopes
Same element, different numbers of neutrons (hence different masses).
Examples: Hydrogen-1 (1p, 0n); Hydrogen-2 (1p, 1n); Uranium-235 (92p, 143n); Uranium-238 (92p, 146n).
Slide 9
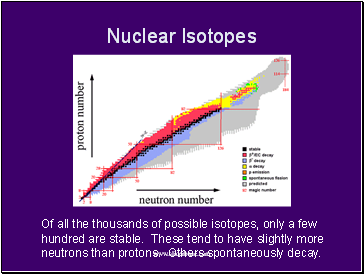
Nuclear Isotopes
Of all the thousands of possible isotopes, only a few hundred are stable. These tend to have slightly more neutrons than protons. Others spontaneously decay.
Slide 10
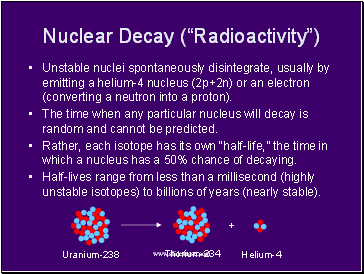
Nuclear Decay (“Radioactivity”)
Unstable nuclei spontaneously disintegrate, usually by emitting a helium-4 nucleus (2p+2n) or an electron (converting a neutron into a proton).
The time when any particular nucleus will decay is random and cannot be predicted.
Contents
- Our Solar System and Others
- Solar System Patterns
- How old is the solar system?
- Nuclear Isotopes
- Nuclear Decay (“Radioactivity”)
- Radioactive Age Dating
- Solar System Patterns
- Formation of the Solar System
- Star formation in the Orion Nebula
- Formation of the Solar System
- Solar System Patterns
Last added presentations
- Static and Kinetic Friction
- Sound
- Mechanics Lecture
- Radiation Safety and Operations
- Upcoming Classes
- Madame Marie Curie
- Ch 9 Nuclear Radiation