An Introduction to MetabolismPage
2
2
to potential energy.
A diver has less potential
energy in the water
than on the platform.
Diving converts
potential energy to
kinetic energy.
A diver has more potential
energy on the platform
than in the water.
Slide 11
The Laws of Energy Transformation
Thermodynamics is the study of energy transformations
A closed system, such as that approximated by liquid in a thermos, is isolated from its surroundings
In an open system, energy and matter can be transferred between the system and its surroundings
Organisms are open systems
Slide 12
The First Law of Thermodynamics
According to the first law of thermodynamics, the energy of the universe is constant:
– Energy can be transferred and transformed, but it cannot be created or destroyed
The first law is also called the principle of conservation of energy
Slide 13
The Second Law of Thermodynamics
During every energy transfer or transformation, some energy is unusable, and is often lost as heat
According to the second law of thermodynamics:
– Every energy transfer or transformation increases the entropy (disorder) of the universe
Slide 14
Fig. 8-3
(a) First law of thermodynamics
(b) Second law of thermodynamics
Chemical
energy
Heat
CO2
H2O
+
Slide 15
Living cells unavoidably convert organized forms of energy to heat
Spontaneous processes occur without energy input; they can happen quickly or slowly
For a process to occur without energy input, it must increase the entropy of the universe
Slide 16
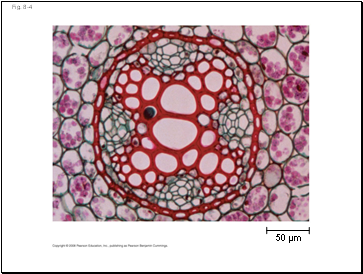
Biological Order and Disorder
Cells create ordered structures from less ordered materials
Organisms also replace ordered forms of matter and energy with less ordered forms
Energy flows into an ecosystem in the form of light and exits in the form of heat
Slide 17
Fig. 8-4
50 µm
Slide 18
The evolution of more complex organisms does not violate the second law of thermodynamics
Entropy (disorder) may decrease in an organism, but the universe’s total entropy increases
Slide 19
Concept 8.2: The free-energy change of a reaction tells us whether or not the reaction occurs spontaneously
Contents
- The Energy of Life
- Organization of the Chemistry of Life into Metabolic Pathways
- Forms of Energy
- The Laws of Energy Transformation
- The First Law of Thermodynamics
- The Second Law of Thermodynamics
- Biological Order and Disorder
- Free-Energy Change, ∆G
- Free Energy, Stability, and Equilibrium
- Free Energy and Metabolism
- Equilibrium and Metabolism
- The Structure and Hydrolysis of ATP
- How ATP Performs Work
- The Regeneration of ATP
- The Activation Energy Barrier
- How Enzymes Lower the EA Barrier
- Substrate Specificity of Enzymes
- Catalysis in the Enzyme’s Active Site
- Effects of Local Conditions on Enzyme Activity
- Cofactors
- Enzyme Inhibitors
- Allosteric Regulation of Enzymes
- Allosteric Activation and Inhibition
- Identification of Allosteric Regulators
- Feedback Inhibition
- Specific Localization of Enzymes Within the Cell
- You should now be able to
Last added presentations
- Solar Thermal Energy
- History of Modern Astronomy
- Mechanics Lecture
- Soil and Plant Nutrition
- Sensory and Motor Mechanisms
- Space Radiation
- Simulation at NASA for the Space Radiation Effort