Organic ChemistryPage
4
4
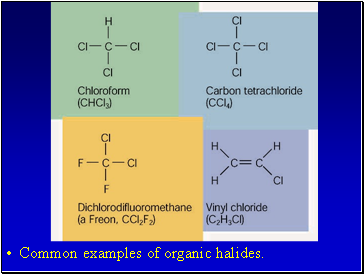
Halogens (F2, Cl2, Br2, I2,) can all add to a hydrocarbon to form am alkyl halide.
When naming the halogen the –ine ending is replaced by –o
Fluorine becomes fluoro
Chlorine becomes chloro
Bromine becomes bromo
Iodine becomes iodo
Slide 28
Common examples of organic halides.
Slide 29
Alkenes can also add to each other in an addition reaction to form long chains of carbon compounds.
this is called polymerization
The atom or group of atoms that are added to the hydrocarbon are called functional groups.
Functional groups usually have multiple bonds or lone pairs of electrons that make them very reactive.
Slide 30
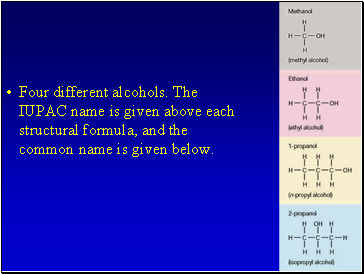
Alcohols
An alcohol has a hydrogen replaced by a hydroxyl (-OH) group.
The name of the hydrocarbon that was substituted determines the name of the alcohol.
The alcohol is named using the hydrocarbon name and adding the suffix –ol.
If methane is substituted with an OH group it becomes methanol
If a pentane group is substituted with an OH group it is pentanol.
For alcohols with more than two carbon atoms we need the number the chain so as to keep the alcohol group as low as possible.
Slide 31
Four different alcohols. The IUPAC name is given above each structural formula, and the common name is given below.
Slide 32
Gasoline is a mixture of hydrocarbons (C8H18 for example) that contain no atoms of oxygen. Gasohol contains ethyl alcohol, C2H5OH, which does contain oxygen. The addition of alcohol to gasoline, therefore, adds oxygen to the fuel. Since carbon monoxide forms when there is an insufficient supply of oxygen, the addition of alcohol to gasoline helps cut down on carbon monoxide emissions. An atmospheric inversion, with increased air pollution, is likely during the dates shown on the pump, so that is when the ethanol is added.
Slide 33
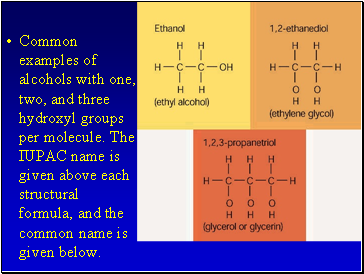
The OH group is polar and short chain alcohols are soluble in both nonpolar alkanes and water.
If an alcohol contains two OH groups it is a diol (sometimes called a glycol).
An alcohol with three OH groups is called a triol (sometimes called a glycerol).
Slide 34
Common examples of alcohols with one, two, and three hydroxyl groups per molecule. The IUPAC name is given above each structural formula, and the common name is given below.
Slide 35
Ethers, Aldehydes, and Ketones
Contents
- Organic Chemistry
- Hydrocarbons
- Petroleum
- Hydrocarbon Derivatives
- Alcohols
- Ethers, Aldehydes, and Ketones
- Organic Acids and Esters
- Organic Compounds of Life
- Proteins
- Carbohydrates
- Fats and Oils
- Synthetic Polymers
- Polymers
Last added presentations
- Newton’s law of universal gravitation
- History of Modern Astronomy
- Newton's laws of motion
- Practical Applications of Solar Energy
- Newton's Laws
- Madame Marie Curie
- Resource Acquisition and Transport in Vascular Plants