The SpectrumPage
4
4
An answer to an old question
From this work B&K understood what the Fraunhofer lines were
‘the vapour of table salt absorbs the same lines which it also emits. These lines are identical with solar Fraunhofer lines’.
So, there is a connection between emission and absorption!
And stars??
Warning! Science Content Follows!
Slide 31
Every element (and molecules too) emits a unique set of spectral lines
It’s like every chord on the piano has a unique set of pitches
But two questions come to mind:
Why does each element emit a unique set of spectral lines?
Why did it take so long for scientists to concertedly look for these lines?
Slide 32
Second Answer First
At the time of Newton’s death in 1727, only 14 of the 114 elements had been identified
Even by 1800, only 32 elements had been isolated
But by 1859, the time of Bunsen and Kirchhoff’s paper, known elements were so numerous that chemists were eager to qualify and quantify them
And, of course, Astronomers weren’t interested at all
Slide 33
Now, the First Answer
This interesting phenomenon, seemingly pertinent only to chemical identification, was one of the motivating forces for a major change in Physics in the 19th and 20th Centuries
“Clouds over Physics”
“Electromagnetic Catastrophe”
Slide 34
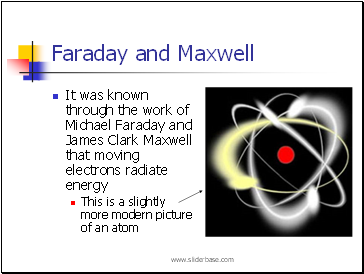
Faraday and Maxwell
It was known through the work of Michael Faraday and James Clark Maxwell that moving electrons radiate energy
This is a slightly more modern picture of an atom
Slide 35
But the (19th C) Problem is:
If an electron is continually buzzing around an atom, it is constantly giving off energy
If it is constantly giving off energy, its path would decay and the electron would spiral into the nucleus
The electron would emit the entire spectrum as it spins down, not discrete colors
And atoms and therefore matter everywhere would collapse
Slide 36
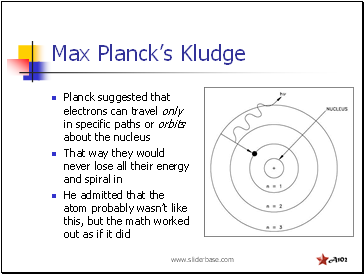
Max Planck’s Kludge
Planck suggested that electrons can travel only in specific paths or orbits about the nucleus
That way they would never lose all their energy and spiral in
He admitted that the atom probably wasn’t like this, but the math worked out as if it did
Slide 37
But Atoms Do Work This Way!
Slide 38
Orbits and Spectra
Contents
- Light and Sound
- If the Rainbow was a Piano…
- Isaac Newton
- William Herschel
- Prisms
- Joseph von Fraunhofer
- What are these lines?
- However, the game was afoot
- Earth-Sun connection
- Physics and Chemistry Unite
- Max Planck’s Kludge
- Orbits and Spectra
- A Powerful Technique
- Kirchhoff’s Laws
- Epiphany
- Other Stellar Mysteries
- Margaret Lindsay Huggins (1848-1915)
- The Modern Variety of Data
- Spin
Last added presentations
- Solar Energy
- Static and Kinetic Friction
- History of Modern Astronomy
- Madame Marie Curie
- Sound
- Friction
- Space Radiation