Thermal EnergyPage
2
2
c varies slightly with temperature
(units of J / kgoC)
Slide 6
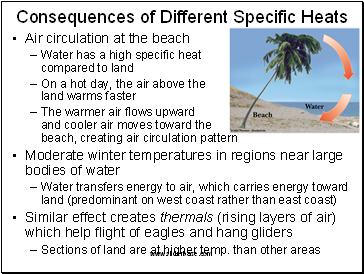
Consequences of Different Specific Heats
Air circulation at the beach
Water has a high specific heat compared to land
On a hot day, the air above the land warms faster
The warmer air flows upward and cooler air moves toward the beach, creating air circulation pattern
Moderate winter temperatures in regions near large bodies of water
Water transfers energy to air, which carries energy toward land (predominant on west coast rather than east coast)
Similar effect creates thermals (rising layers of air) which help flight of eagles and hang gliders
Sections of land are at higher temp. than other areas
Slide 7
Calorimetry
Calorimetry means “measuring heat”
In practice, it is a technique used to measure specific heat
Technique involves:
Raising temperature of object(s) to some value
Place object(s) in vessel containing cold water of known mass and temperature
Measure temperature of object(s) + water after equilibrium is reached
A calorimeter is a vessel providing good insulation that allows a thermal equilibrium to be achieved between substances without any energy loss to the environment (styrofoam cup or thermos with lid)
Conservation of energy requires that:
(Q > 0 (< 0) when energy is gained (lost))
Slide 8
Example Problem #11.17
Solution (details given in class):
80 g
An aluminum cup contains 225 g of water and a 40-g copper stirrer, all at 27°C. A 400-g sample of silver at an initial temperature of 87°C is placed in the water. The stirrer is used to stir the mixture until it reaches its final equilibrium temperature of 32°C. Calculate the mass of the aluminum cup.
Slide 9
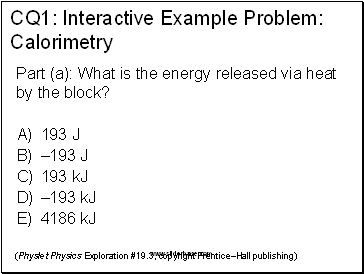
CQ1: Interactive Example Problem: Calorimetry
(Physlet Physics Exploration #19.3, copyright Prentice–Hall publishing)
Part (a): What is the energy released via heat by the block?
193 J
–193 J
193 kJ
–193 kJ
4186 kJ
Slide 10
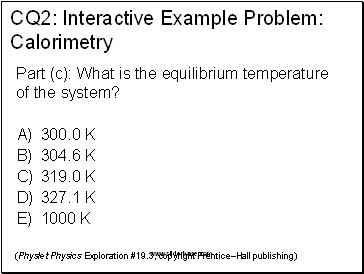
CQ2: Interactive Example Problem: Calorimetry
(Physlet Physics Exploration #19.3, copyright Prentice–Hall publishing)
Part (c): What is the equilibrium temperature of the system?
300.0 K
304.6 K
319.0 K
327.1 K
1000 K
Slide 11
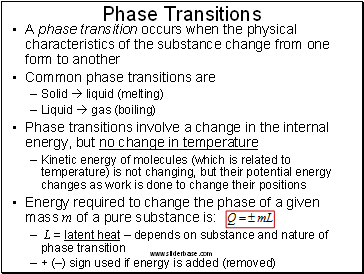
Phase Transitions
A phase transition occurs when the physical characteristics of the substance change from one form to another
Contents
- Heat and Internal Energy
- Units of Heat
- More About Heat
- Heat Transfer Simulation
- Specific Heat
- Consequences of Different Specific Heats
- Calorimetry
- Phase Transitions
- Evaporation and Condensation
- Conduction
- Home Insulation
- Convection
- Thermal Radiation
- Applications of Thermal Radiation
- Resisting Energy Transfer
- Global Warming
Last added presentations
- Newton’s law of universal gravitation
- Soil and Plant Nutrition
- Waves & Sound
- Buoyancy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Geophysical Concepts, Applications and Limitations
- Direct heat utilization of geothermal energy