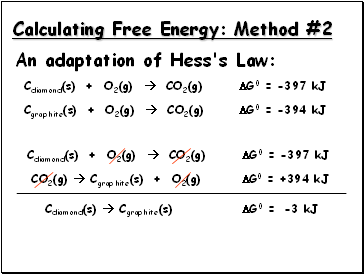Spontaneity, Entropy, and Free EnergyPage
2
2
G0 = H0 - TS0
Calculating Free Energy Method #1
Slide 10
Calculating Free Energy: Method #2
An adaptation of Hess's Law:
Cdiamond(s) + O2(g) CO2(g) G0 = -397 kJ
Cgraphite(s) + O2(g) CO2(g) G0 = -394 kJ
CO2(g) Cgraphite(s) + O2(g) G0 = +394 kJ
Cdiamond(s) Cgraphite(s)
G0 =
Cdiamond(s) + O2(g) CO2(g) G0 = -397 kJ
-3 kJ
Slide 11
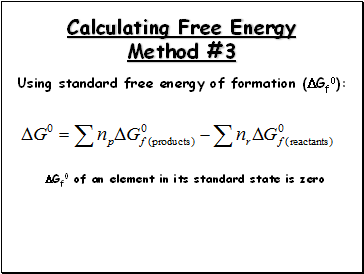
Calculating Free Energy Method #3
Using standard free energy of formation (Gf0):
Gf0 of an element in its standard state is zero
Slide 12
The Dependence of Free Energy on Pressure
Enthalpy, H, is not pressure dependent
Entropy, S
entropy depends on volume, so it also depends on pressure
Slarge volume > Ssmall volume
Slow pressure > Shigh pressure
Slide 13
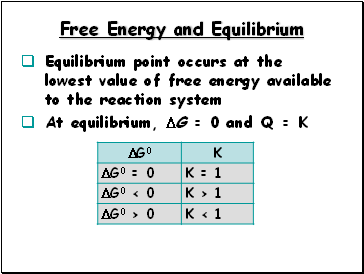
Free Energy and Equilibrium
Equilibrium point occurs at the lowest value of free energy available to the reaction system
At equilibrium, G = 0 and Q = K
Slide 14
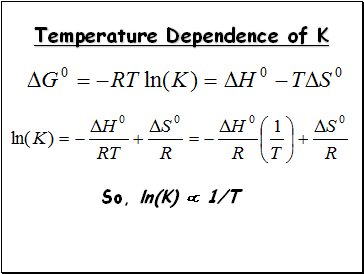
Temperature Dependence of K
So, ln(K) 1/T
Slide 15
Free Energy and Work
The maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy
The amount of work obtained is always less than the maximum
Henry Bent's First Two Laws of Thermodynamics
First law: You can't win, you can only break even
Second law: You can't break even
1 2
Contents
- Spontaneous Processes and Entropy
- Entropy (S)
- Positional Entropy
- Second Law of Thermodynamics
- Calculating Entropy Change in a Reaction
- Standard Free Energy Change
- The Dependence of Free Energy on Pressure
- Free Energy and Equilibrium
- Temperature Dependence of K
- Free Energy and Work
Last added presentations
- Friction
- Sound
- Health Physics
- Newton's laws of motion
- Static and Kinetic Friction
- Upcoming Classes
- Newton’s laws of motion