VolumetricPage
3
3
A solution is prepared by dissolving a solute in a solvent.
Slide 17
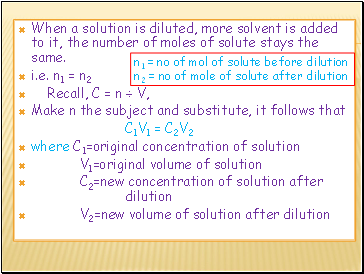
When a solution is diluted, more solvent is added to it, the number of moles of solute stays the same.
i.e. n1 = n2
Recall, C = n ÷ V,
Make n the subject and substitute, it follows that
C1V1 = C2V2
where C1=original concentration of solution
V1=original volume of solution
C2=new concentration of solution after dilution
V2=new volume of solution after dilution
n1 = no of mol of solute before dilution
n2 = no of mole of solute after dilution
Slide 18
To calculate the new concentration (C2) of a solution given its new volume (V2) and its original concentration (C1) and original volume (V1).
Note: V2 = V1 + vol. of water added.
Slide 19
Examples
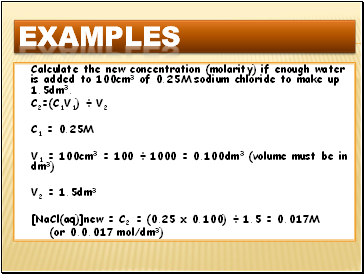
Calculate the new concentration (molarity) if enough water is added to 100cm3 of 0.25M sodium chloride to make up 1.5dm3.
C2=(C1V1) ÷ V2
C1 = 0.25M
V1 = 100cm3 = 100 ÷ 1000 = 0.100dm3 (volume must be in dm3)
V2 = 1.5dm3
[NaCl(aq)]new = C2 = (0.25 x 0.100) ÷ 1.5 = 0.017M
(or 0.0.017 mol/dm3)
Slide 20
More
If 280cm3 of a 3moldm-3 sodium hydroxide solution is diluted to give 0.7moldm-3 soln.
What is the vol. of the resulting diluted solution?
What is the vol. of distilled water added to the original soln.?
Slide 21
Letís do it
V1 = 280cm3 ,C1 = 3moldm-3 ,C2 = 0.7moldm-3
V2 = ?
C1V1 = C2V2
V2 = 3 X 280 = 1200cm3 0.7
To know the vol. of distill water added
V2 = V1 + vol. of distill water added.
vol. of distill water added.= 1200 Ė 280
= 920cm3
Slide 22
One more!
Calculate the vol. of a 12.0moldm-3 HCl that should be diluted with distilled water to obtain 1.0dm3 of a 0.05moldm-3 HCl.
Soln.
C1 = 12moldm-3, V1 = ?
C2 = 0.05moldm-3 , V2 = 1.0dm3
Iíve done my own part, do yours!
Slide 23
PRActice problems
Slide 24
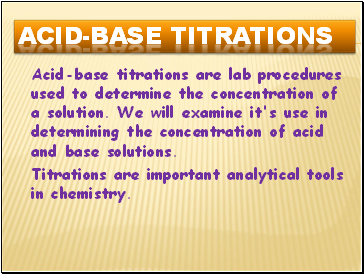
Acid-Base Titrations
Acid-base titrations are lab procedures used to determine the concentration of a solution. We will examine it's use in determining the concentration of acid and base solutions.
Titrations are important analytical tools in chemistry.
Slide 25
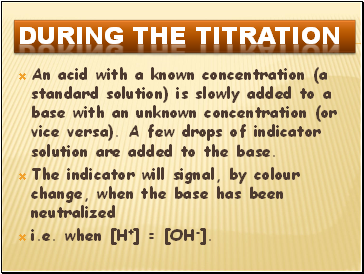
During the titration
Contents
- Introduction
- Definition of terms
- Relationship between molar conc & mass conc
- Concentration of solution
- Solved problems involved concentration
- Alternatively
- Principle of dillution (dillution factor)
- Acid-Base Titrations
- During the titration
- At the end point
- Volumetric apparatus
- Titration Procedure
- How do you know when you are reaching the endpoint?
- Precautions during titration
- Recording in titration
- Indicator Selection for Titrations
- Titration Calculations
- Details of the theory behind the calculations
- The theory
- Tips on solving the problem
- Letís solve it together
Last added presentations
- Sound
- Radioactivity and Nuclear Reactions
- Buoyancy
- Thermal Energy
- Motion
- Newton's Laws
- Geophysical Concepts, Applications and Limitations