VolumetricPage
4
4
An acid with a known concentration (a standard solution) is slowly added to a base with an unknown concentration (or vice versa). A few drops of indicator solution are added to the base.
The indicator will signal, by colour change, when the base has been neutralized
i.e. when [H+] = [OH-].
Slide 26
At the end point
At that point - called the equivalence point or end point - the titration is stopped. By knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to determine the concentration of the other solution.
Slide 27
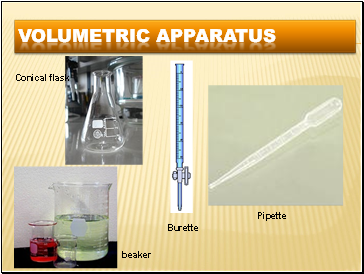
Volumetric apparatus
Pipette
Burette
Conical flask
beaker
Slide 28
Titration Procedure
Rinse 20 or 25cm3 pipette with the base solutions.
Using the pipette, accurately measure 20 or 25cm3 of the base into a clean conical flask.
Add 2 or 3 drops of a suitable indicator to the base in the flask.
Pour the acid into the burette using a funnel.
Adjust the tap to expel air bubbles and then take the initial burette reading.
Slide 29
Titration Procedure
Place the conical flask on a white tile under the burette.
Run the solution gradually from the burette into the conical flask and swirl the flask along.
Continue the addition with swirling until the end point is reached.
Slide 30
How do you know when you are reaching the endpoint?
The indicator will begin to show a change in colour. Swirling the flask will cause the colour to disappear.
ENDPOINT IS REACHED AS SOON AS THE COLOUR CHANGE IN PERMANENT.
ONE DROP WILL DO IT - once the colour change has occurred, stop adding additional acid
Slide 31
Warning!
Do NOT continue adding until you get a deep colour change - you just want to get a permanent colour change that does not disappear upon mixing.
NOTE:
If a pH meter is used instead of an indicator, endpoint will be reached when there is a sudden change in pH.
Slide 32
Then,
Record the burette reading. The difference between the final and the initial burette readings gives the volume of the acid used.
The titration should be repeated two or more times and the results averaged.
Slide 33
Precautions during titration
Contents
- Introduction
- Definition of terms
- Relationship between molar conc & mass conc
- Concentration of solution
- Solved problems involved concentration
- Alternatively
- Principle of dillution (dillution factor)
- Acid-Base Titrations
- During the titration
- At the end point
- Volumetric apparatus
- Titration Procedure
- How do you know when you are reaching the endpoint?
- Precautions during titration
- Recording in titration
- Indicator Selection for Titrations
- Titration Calculations
- Details of the theory behind the calculations
- The theory
- Tips on solving the problem
- Letís solve it together
Last added presentations
- Motion
- Newton's laws of motion
- Radioactivity and Nuclear Reactions
- Upcoming Classes
- Heat-Energy on the Move
- Direct heat utilization of geothermal energy
- Sound