Acid, bases and saltsPage
2
2
HNO3 (aq) = nitric acid
(polyatomic ion) -ide +ic acid
HCN (aq) = cyanic acid
(polyatomic ion) -ite +ous acid
HNO2 (aq) = nitrous acid
Slide 9
Formula Writing of Acids
Acids formulas get written like any other. Write the H+1 first, then figure out what the negative ion is based on the name. Cancel out the charges to write the formula. Don’t forget the (aq) after it…it’s only an acid if it’s in water!
Carbonic acid: H+1 and CO3-2 = H2CO3 (aq)
Chlorous acid: H+1 and ClO2-1 = HClO2 (aq)
Hydrobromic acid: H+1 and Br-1 = HBr (aq)
Hydronitric acid:
Slide 10
Properties of Bases
Bases react with fats to form soap and glycerol. This process is called saponification.
Bases have a pH of more than 7.
Dilute solutions of bases taste bitter.
Bases turn phenolphthalein PINK, litmus BLUE and bromthymol blue BLUE.
Bases neutralize acids.
Bases are formed when alkali metals or alkaline earth metals react with water. The words “alkali” and “alkaline” mean “basic”, as opposed to “acidic”.
Slide 11
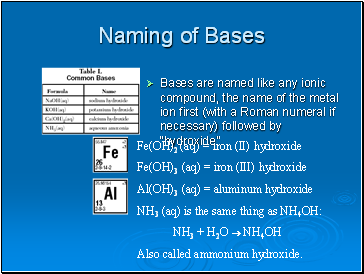
Naming of Bases
Bases are named like any ionic compound, the name of the metal ion first (with a Roman numeral if necessary) followed by “hydroxide”.
Fe(OH)2 (aq) = iron (II) hydroxide
Fe(OH)3 (aq) = iron (III) hydroxide
Al(OH)3 (aq) = aluminum hydroxide
NH3 (aq) is the same thing as NH4OH:
NH3 + H2O NH4OH
Also called ammonium hydroxide.
Slide 12
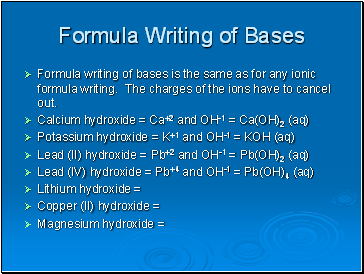
Formula Writing of Bases
Formula writing of bases is the same as for any ionic formula writing. The charges of the ions have to cancel out.
Calcium hydroxide = Ca+2 and OH-1 = Ca(OH)2 (aq)
Potassium hydroxide = K+1 and OH-1 = KOH (aq)
Lead (II) hydroxide = Pb+2 and OH-1 = Pb(OH)2 (aq)
Lead (IV) hydroxide = Pb+4 and OH-1 = Pb(OH)4 (aq)
Lithium hydroxide =
Copper (II) hydroxide =
Magnesium hydroxide =
Slide 13
Physical Properties of Acids & Bases
ACIDS
Acids taste sour (e.g. vinegar, lemon juice).
Acids are harmful to living cells.
Aqueous solutions of all acids contain hydrogen ions.
Acid turns blue litmus red.
Strong acids are corrosive.
BASES
Alkalis are taste bitter
Strong alkalis are corrosive.
Aqueous solutions of all alkalis contain hydroxide ion.
Alkalis turns red litmus blue.
Soapy touch.
Slide 14
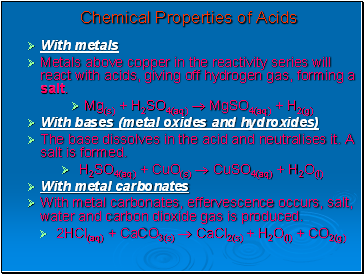
Chemical Properties of Acids
With metals
Metals above copper in the reactivity series will react with acids, giving off hydrogen gas, forming a salt.
Contents
- Acids, Bases & Salts
- Terms
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- Formation of Hydronium ion( H30+).
- Uses of Acids
- Chemical Properties of Bases
- Chemical Properties of Bases
- Types of Oxides
- Periodic trends in oxides
- Salts
- Methods of making Soluble Salts
- Making Insoluble Salts
- Precipitation reaction
- Types of Salts
- Hydrated & anhydrous salts
- Uses of salts
- Self Ionization of Water
- The pH Scale
- Indicators.
- pH Graph
- Ionic equations
- Scheme for ionic equation
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Space Radiation
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Waves & Sound
- Mechanics Lecture
- Direct heat utilization of geothermal energy
- Resource Acquisition and Transport in Vascular Plants