Acid, bases and saltsPage
7
7
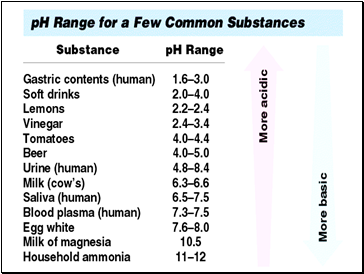
A pH of 4 is 10 times more acidic than a pH of 5.
A pH of 12 is 100 times more basic than a pH of 10.
Slide 42
Slide 43
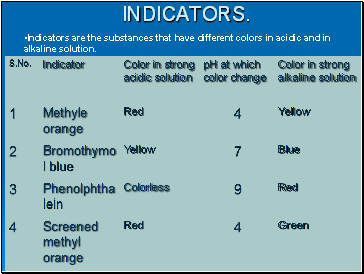
Indicators.
Indicators are the substances that have different colors in acidic and in alkaline solution.
Slide 44
Slide 45
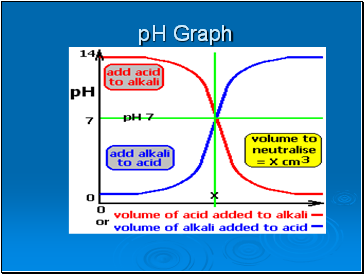
pH Graph
Slide 46
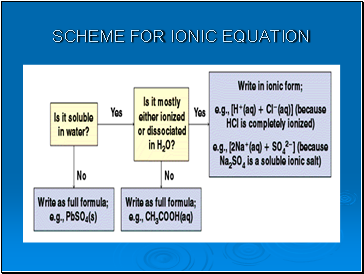
Ionic equations
In many reactions only certain ions change their 'chemical state' but other ions remain in exactly the same original physical and chemical state.
The ions that do not change are called 'spectator ions'.
The ionic equation represents the 'actual' chemical change and omits the spectator ions.
To write a net ionic equation:
Write a balanced molecular equation.
Rewrite the equation showing the ions that form in solution when each soluble electrolyte dissociates into its component ions. Only dissolved strong electrolytes are written in ionic form.
Identify and cancel the spectator ions that occur unchanged on both sides of the equation.
Write correct state symbols.
Slide 47
Scheme for ionic equation
Slide 48
THE END
Contents
- Acids, Bases & Salts
- Terms
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- Formation of Hydronium ion( H30+).
- Uses of Acids
- Chemical Properties of Bases
- Chemical Properties of Bases
- Types of Oxides
- Periodic trends in oxides
- Salts
- Methods of making Soluble Salts
- Making Insoluble Salts
- Precipitation reaction
- Types of Salts
- Hydrated & anhydrous salts
- Uses of salts
- Self Ionization of Water
- The pH Scale
- Indicators.
- pH Graph
- Ionic equations
- Scheme for ionic equation
Last added presentations
- Upcoming Classes
- Thermal Energy
- Solar Energy
- Sensory and Motor Mechanisms
- Gravitation
- Sound
- Newton’s law of universal gravitation