AtomsPage
2
2
electron orbits
Slide 8
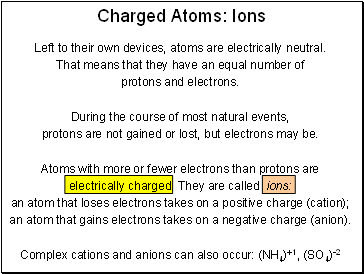
Charged Atoms: Ions
Left to their own devices, atoms are electrically neutral.
That means that they have an equal number of
protons and electrons.
During the course of most natural events,
protons are not gained or lost, but electrons may be.
Atoms with more or fewer electrons than protons are
electrically charged. They are called ions:
an atom that loses electrons takes on a positive charge (cation);
an atom that gains electrons takes on a negative charge (anion).
Complex cations and anions can also occur: (NH4)+1, (SO4)-2
Slide 9
Atomic Number
We distinguish one element from another on the basis of the atomic number, which is the number of protons.
So, an atom of any element can have a variable number of electrons and neutrons, but given the number of protons, the fundamental properties of the element are unchanged.
This is the basis for Dmitri Mendeleevís organization of the
Periodic Table of the Elements.
The table is a way of organizing elements
on physical grounds,
but also serves to group elements with consistent chemical properties.
Slide 10
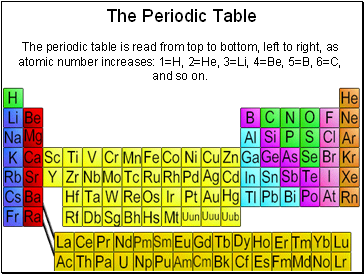
The Periodic Table
The periodic table is read from top to bottom, left to right, as atomic number increases: 1=H, 2=He, 3=Li, 4=Be, 5=B, 6=C, and so on.
Slide 11
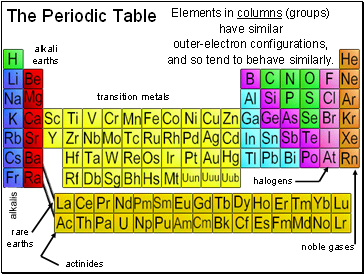
Elements in columns (groups) have similar
outer-electron configurations,
and so tend to behave similarly.
The Periodic Table
alkalis
alkali earths
rare earths
halogens
noble gases
transition metals
actinides
Slide 12
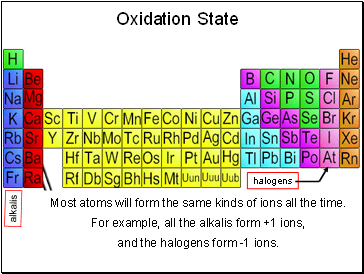
Oxidation State
Most atoms will form the same kinds of ions all the time.
For example, all the alkalis form +1 ions,
and the halogens form -1 ions.
alkalis
halogens
Slide 13
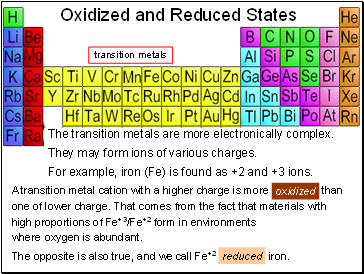
Oxidized and Reduced States
A transition metal cation with a higher charge is more oxidized than
one of lower charge. That comes from the fact that materials with
high proportions of Fe+3/Fe+2 form in environments
where oxygen is abundant.
The opposite is also true, and we call Fe+2 reduced iron.
transition metals
The transition metals are more electronically complex.
They may form ions of various charges.
For example, iron (Fe) is found as +2 and +3 ions.
oxidized
reduced
Slide 14
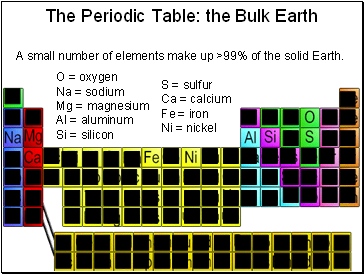
The Periodic Table: the Bulk Earth
Contents
- The Building Blocks
- The Stuff That Makes Atoms
- Maintaining Neutrality
- Electronic and Nuclear Properties
- Size of Nuclei
- The Spacious Atom
- Electrons in Orbit
- Charged Atoms: Ions
- Atomic Number
- The Periodic Table
- Oxidation State
- Oxidized and Reduced States
- The Periodic Table: the Bulk Earth
- The Periodic Table: the Crust
- Atomic Weight: Itís all in the Nucleus
- Isotopes
- Radioactive or Stable?
- Stable and Radioactive Isotopes
- Radioactivity Inside You
- Chemical Bonds
- Ionic Bonds
- Covalent Bonds: Electron Sharing
- Alternative Bonds
- Chemical Reactions: Achieving Stability
- What is a Mineral?
- Stability
- Stability and Metastability
Last added presentations
- Newtonís Law of Gravity
- Solar Energy
- Upcoming Classes
- Simulation at NASA for the Space Radiation Effort
- Direct heat utilization of geothermal energy
- Mechanics Lecture
- Heat-Energy on the Move