DensityPage
1
1
Slide 1
Density
Q) Which weighs more:-
A kilogram of feathers or a kilogram of iron?
Slide 2
What is Density?
Density is the Mass per unit Volume
Wood
Water
Iron
1 cm3
1 cm3
1 cm3
If you take the same volume of different substances, then they will weigh different amounts.
0.50 g
1.00 g
8.00 g
Q) Which has the greatest mass and therefore the most dense?
IRON
Slide 3
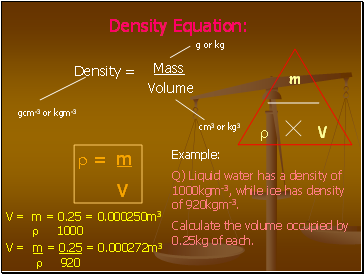
Density Equation
Density =
Mass
Volume
g or kg
cm3 or kg3
gcm-3 or kgm-3
= m
V
Example:
Q) Liquid water has a density of 1000kgm-3, while ice has density of 920kgm-3.
Calculate the volume occupied by 0.25kg of each.
:
V = m = 0.25 = 0.000250m3
1000
V = m = 0.25 = 0.000272m3
920
Slide 4
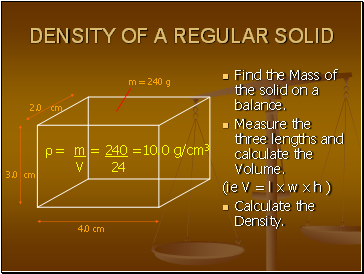
Density of a regular solid
Find the Mass of the solid on a balance.
Measure the three lengths and calculate the Volume.
(ie V = l x w x h )
Calculate the Density.
4.0 cm
2.0 cm
3.0 cm
= m = 240 =10.0 g/cm3
V 24
m = 240 g
Slide 5
Slide 6
Slide 7
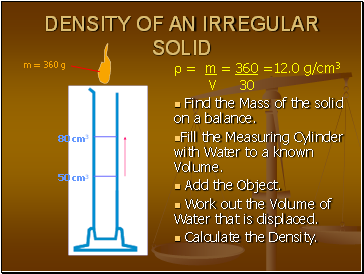
DENSITY OF AN IRREGULAR SOLID
Find the Mass of the solid on a balance.
Fill the Measuring Cylinder with Water to a known Volume.
Add the Object.
Work out the Volume of Water that is displaced.
Calculate the Density.
50 cm3
80 cm3
m = 360 g
= m = 360 =12.0 g/cm3
V 30
Slide 8
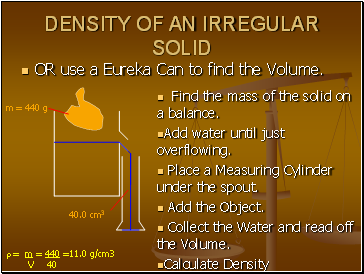
DENSITY OF AN IRREGULAR SOLID
OR use a Eureka Can to find the Volume.
Find the mass of the solid on a balance.
Add water until just overflowing.
Place a Measuring Cylinder under the spout.
Add the Object.
Collect the Water and read off the Volume.
Calculate Density
m = 440 g
40.0 cm3
= m = 440 =11.0 g/cm3
V 40
Slide 9
Slide 10
Density of a liquid
Find the Mass of an empty Measuring Cylinder.
Add a certain Volume of Liquid.
Find the Mass of the Measuring Cylinder and Liquid
Calculate the Mass of Liquid.
How?
Calculate Density of Liquid.
1 2
Contents
- Density
- What is Density?
- Density Equation
- Density of a regular solid
- Density of a liquid
- Density of a gas
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Thermal Energy
- Solar Energy
- Radiation
- Motion
- Practical Applications of Solar Energy