Atomic Structure and PeriodicityPage
4
4
= mathematical operator
E = total energy of the atom
A specific wave function is often called an orbital.
This equation is based on operators – not simple algebra. This is a mathematical concept you will not have dealt with yet.
Slide 24
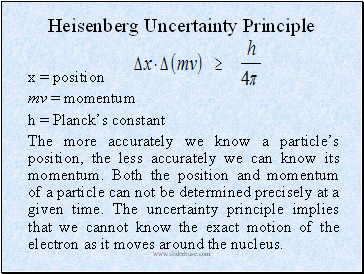
Heisenberg Uncertainty Principle
x = position
mv = momentum
h = Planck’s constant
The more accurately we know a particle’s position, the less accurately we can know its momentum. Both the position and momentum of a particle can not be determined precisely at a given time. The uncertainty principle implies that we cannot know the exact motion of the electron as it moves around the nucleus.
Slide 25
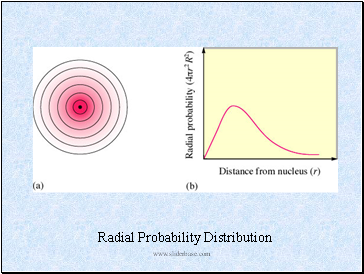
Radial Probability Distribution
Slide 26
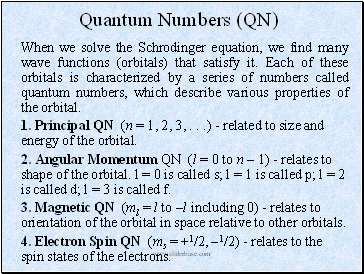
Quantum Numbers (QN)
When we solve the Schrodinger equation, we find many wave functions (orbitals) that satisfy it. Each of these orbitals is characterized by a series of numbers called quantum numbers, which describe various properties of the orbital.
1. Principal QN (n = 1, 2, 3, . . .) - related to size and energy of the orbital.
2. Angular Momentum QN (l = 0 to n 1) - relates to shape of the orbital. l = 0 is called s; l = 1 is called p; l = 2 is called d; l = 3 is called f.
3. Magnetic QN (ml = l to l including 0) - relates to orientation of the orbital in space relative to other orbitals.
4. Electron Spin QN (ms = +1/2, 1/2) - relates to the spin states of the electrons.
Slide 27
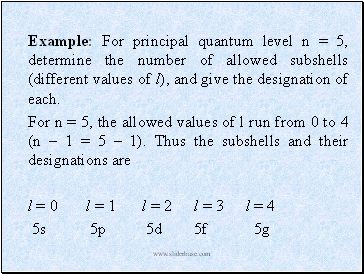
Example: For principal quantum level n = 5, determine the number of allowed subshells (different values of l), and give the designation of each.
For n = 5, the allowed values of l run from 0 to 4 (n – 1 = 5 – 1). Thus the subshells and their designations are
l = 0 l = 1 l = 2 l = 3 l = 4
5s 5p 5d 5f 5g
Slide 28
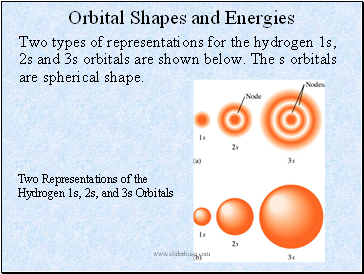
Orbital Shapes and Energies
Two types of representations for the hydrogen 1s, 2s and 3s orbitals are shown below. The s orbitals are spherical shape.
Two Representations of the Hydrogen 1s, 2s, and 3s Orbitals
Slide 29
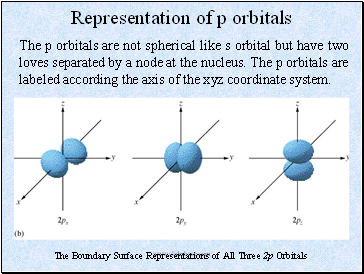
Representation of p orbitals
The p orbitals are not spherical like s orbital but have two loves separated by a node at the nucleus. The p orbitals are labeled according the axis of the xyz coordinate system.
The Boundary Surface Representations of All Three 2p Orbitals
Slide 30
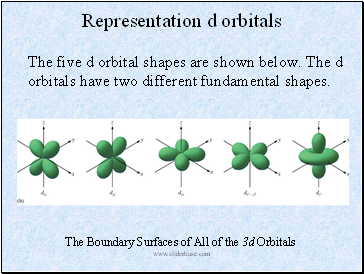
Representation d orbitals
Contents
- Waves
- Planck’s Constant
- Energy and Mass
- Wavelength and Mass
- Atomic Spectrum of Hydrogen
- The Bohr Model
- Quantum Mechanics
- Heisenberg Uncertainty Principle
- Quantum Numbers (QN)
- Orbital Shapes and Energies
- Representation of p orbitals
- Representation d orbitals
- Energy Diagram for Hydrogen Atom
- Pauli Exclusion Principle
- Polyelectronic Atoms
- Aufbau Principle
- Hund’s Rule
- Valence Electrons
- Broad Periodic Table Classifications
- Ionization Energy
- Periodic Trends
- Electron Affinity
- Periodic Trends
- Information Contained in the Periodic Table
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Newton's Laws
- Newton’s Laws of Motion
- Static and Kinetic Friction
- Direct heat utilization of geothermal energy
- Ch 9 Nuclear Radiation
- Solar Thermal Energy