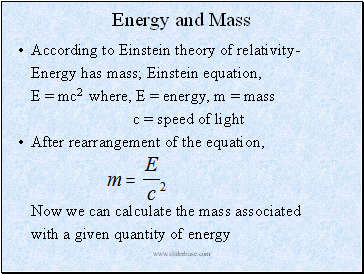Atomic Structure and PeriodicityPage
2
2
A sample of CuCl emitting light at 450 nm can only lose energy in increments of 4.41 x 10-19J, the size of the quantum in this case.
Slide 9
Energy and Mass
According to Einstein theory of relativity-
Energy has mass; Einstein equation,
E = mc2 where, E = energy, m = mass
c = speed of light
After rearrangement of the equation,
Now we can calculate the mass associated
with a given quantity of energy
Slide 10
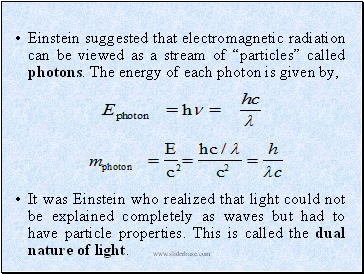
Einstein suggested that electromagnetic radiation can be viewed as a stream of “particles” called photons. The energy of each photon is given by,
It was Einstein who realized that light could not be explained completely as waves but had to have particle properties. This is called the dual nature of light.
Slide 11
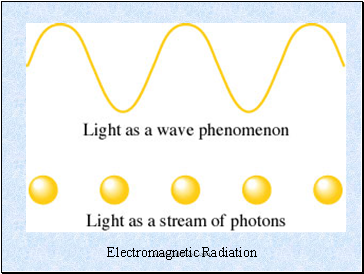
Electromagnetic Radiation
Slide 12
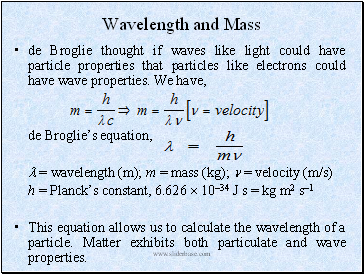
Wavelength and Mass
de Broglie thought if waves like light could have particle properties that particles like electrons could have wave properties. We have,
de Broglie’s equation,
= wavelength (m); m = mass (kg); = velocity (m/s)
h = Planck’s constant, 6.626 1034 J s = kg m2 s1
This equation allows us to calculate the wavelength of a particle. Matter exhibits both particulate and wave properties.
Slide 13
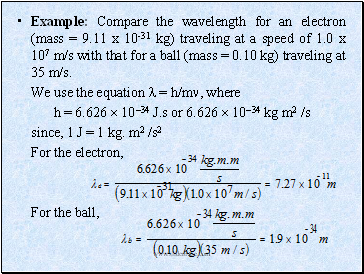
Example: Compare the wavelength for an electron (mass = 9.11 x 10-31 kg) traveling at a speed of 1.0 x 107 m/s with that for a ball (mass = 0.10 kg) traveling at 35 m/s.
We use the equation = h/m, where
h = 6.626 1034 J.s or 6.626 1034 kg m2 /s
since, 1 J = 1 kg. m2 /s2
For the electron,
For the ball,
Slide 14
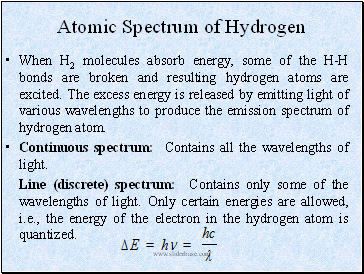
Atomic Spectrum of Hydrogen
When H2 molecules absorb energy, some of the H-H bonds are broken and resulting hydrogen atoms are excited. The excess energy is released by emitting light of various wavelengths to produce the emission spectrum of hydrogen atom.
Continuous spectrum: Contains all the wavelengths of light.
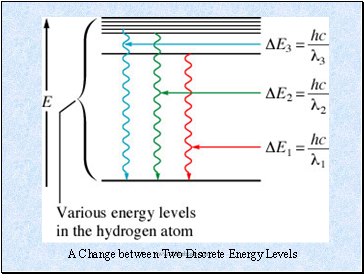
Line (discrete) spectrum: Contains only some of the wavelengths of light. Only certain energies are allowed, i.e., the energy of the electron in the hydrogen atom is quantized.
Slide 15
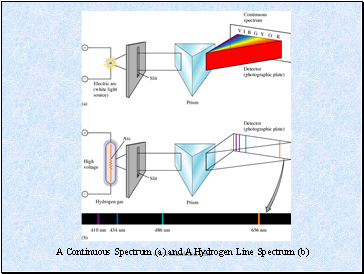
A Continuous Spectrum (a) and A Hydrogen Line Spectrum (b)
Slide 16
Contents
- Waves
- Planck’s Constant
- Energy and Mass
- Wavelength and Mass
- Atomic Spectrum of Hydrogen
- The Bohr Model
- Quantum Mechanics
- Heisenberg Uncertainty Principle
- Quantum Numbers (QN)
- Orbital Shapes and Energies
- Representation of p orbitals
- Representation d orbitals
- Energy Diagram for Hydrogen Atom
- Pauli Exclusion Principle
- Polyelectronic Atoms
- Aufbau Principle
- Hund’s Rule
- Valence Electrons
- Broad Periodic Table Classifications
- Ionization Energy
- Periodic Trends
- Electron Affinity
- Periodic Trends
- Information Contained in the Periodic Table
Last added presentations
- Buoyancy
- Space Radiation
- Radiation Safety and Operations
- History of Modern Astronomy
- Gravitation
- Upcoming Classes
- Sound