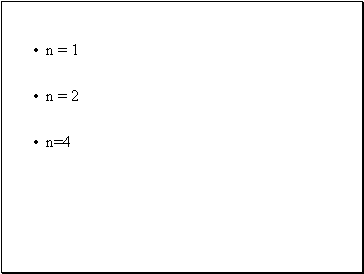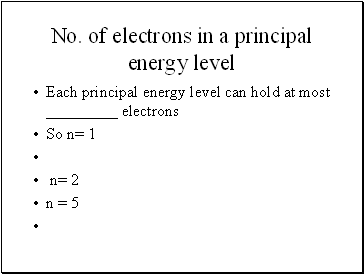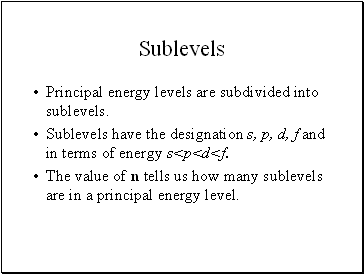Periodictable - QuestionsPage
3
3
The principal energy levels are designated by the quantum no. n.
Allowed values of n:
Each e- in an atom can be found only in certain allowed principal energy levels (shells) (designated by the q. no. n)
Slide 20
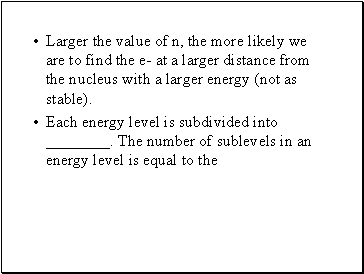
Larger the value of n, the more likely we are to find the e- at a larger distance from the nucleus with a larger energy (not as stable).
Each energy level is subdivided into . The number of sublevels in an energy level is equal to the
Slide 21
n = 1
n = 2
n=4
Slide 22
No. of electrons in a principal energy level
Each principal energy level can hold at most _ electrons
So n= 1
n= 2
n = 5
Slide 23
Sublevels
Principal energy levels are subdivided into sublevels.
Sublevels have the designation s, p, d, f and in terms of energy s<p<d<f.
The value of n tells us how many sublevels are in a principal energy level.
Slide 24
So for n = 1 there is one sublevel . The 1 gives us the principal energy level and the s tells us the type of orbital that is found in that sublevel.
For n =2 we have and sublevels making up that energy level.
For n= 3 we have
For n =4 we have
For n=5 we have
We donít worry about any type of orbital (sublevel) beyond f.
Slide 25
Orbitals
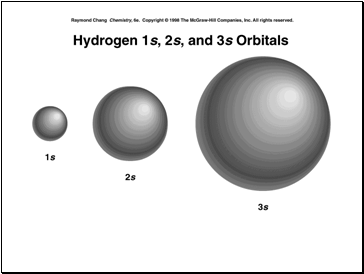
An orbital is a region in space where there is a large probability of finding an electron.
Each orbital can hold at most _ electrons. So an orbital can be
Types of orbitals are designated by the s, p, d, f letters.
Slide 26
The s sublevel is made up of _ orbital shaped like a sphere and can hold at most _ electrons.
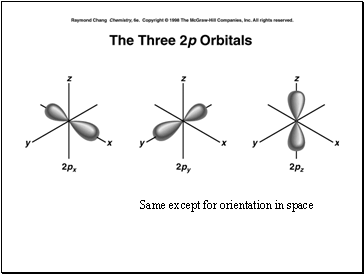
The p sublevel is made up of orbitals. Since each orbital can hold a maximum of 2 electrons, the set of p sublevels can hold a total of _ electrons.
Slide 27
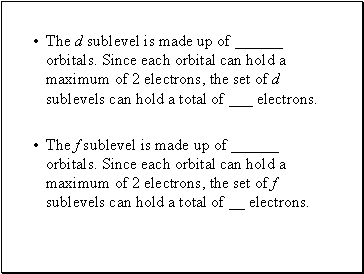
The d sublevel is made up of orbitals. Since each orbital can hold a maximum of 2 electrons, the set of d sublevels can hold a total of _ electrons.
The f sublevel is made up of orbitals. Since each orbital can hold a maximum of 2 electrons, the set of f sublevels can hold a total of electrons.
Slide 28
Slide 29
Contents
- Elements, atoms, ions, and the periodic table
- The periodic law and the periodic table
- Early periodic tables
- Modern periodic table
- Metals and nonmetals
- More info from periodic table
- Electron arrangement and the periodic table
- Principal energy levels (shells)
- Sublevels
- Orbitals
- Electron spin
- What to do with all this info?
- Abbreviated electron configuration
- Valence electrons
- Valence electron configuration for A groups
- The octet rule
- Transition metal cations
- Whatís the ion formed by
- Isoelectronic
- Trends in the periodic table
- Size across a period
- Ion size
- Ionization energy
- Trends in ionization energy
- Electron affinity
- Trends in electron affinities
Last added presentations
- Sensory and Motor Mechanisms
- Solar Energy
- Practical Applications of Solar Energy
- The Effects of Radiation on Living Things
- Friction
- Soil and Plant Nutrition
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal