Rate LawsPage
1
1
Slide 1
Rates and Rate Laws
Slide 2
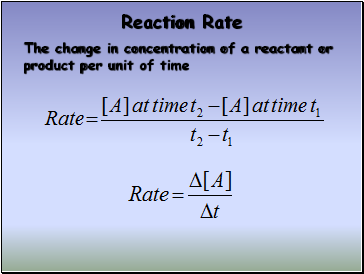
Reaction Rate
The change in concentration of a reactant or product per unit of time
Slide 3
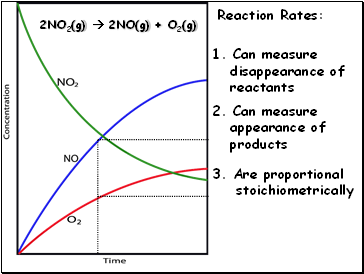
2NO2(g) 2NO(g) + O2(g)
Reaction Rates:
2. Can measure
appearance of
products
1. Can measure
disappearance of
reactants
3. Are proportional
stoichiometrically
Slide 4
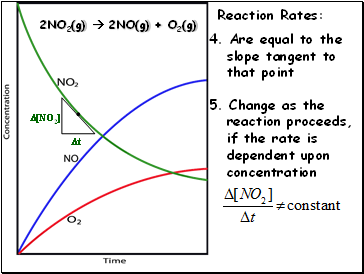
2NO2(g) 2NO(g) + O2(g)
Reaction Rates:
4. Are equal to the
slope tangent to
that point
[NO2]
t
5. Change as the
reaction proceeds,
if the rate is
dependent upon
concentration
Slide 5
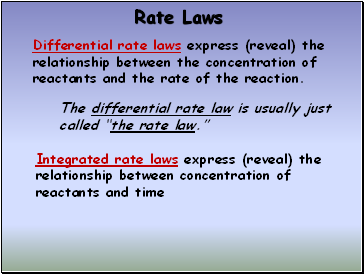
Rate Laws
Differential rate laws express (reveal) the relationship between the concentration of reactants and the rate of the reaction.
Integrated rate laws express (reveal) the relationship between concentration of reactants and time
The differential rate law is usually just called “the rate law.”
Slide 6
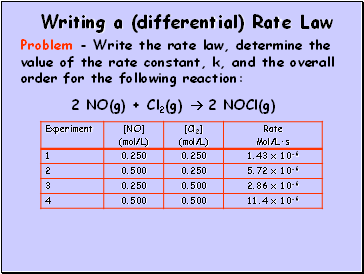
Writing a (differential) Rate Law
2 NO(g) + Cl2(g) 2 NOCl(g)
Problem - Write the rate law, determine the value of the rate constant, k, and the overall order for the following reaction:
Slide 7
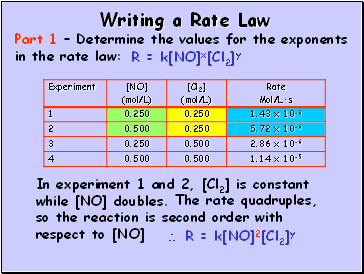
Writing a Rate Law
Part 1 – Determine the values for the exponents in the rate law:
In experiment 1 and 2, [Cl2] is constant while [NO] doubles.
R = k[NO]x[Cl2]y
The rate quadruples, so the reaction is second order with respect to [NO]
R = k[NO]2[Cl2]y
Slide 8
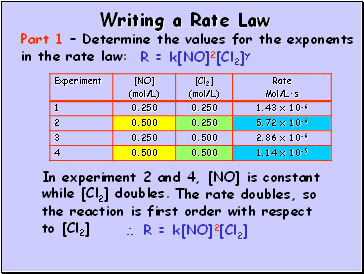
Writing a Rate Law
Part 1 – Determine the values for the exponents in the rate law:
R = k[NO]2[Cl2]y
In experiment 2 and 4, [NO] is constant while [Cl2] doubles.
The rate doubles, so the reaction is first order with respect to [Cl2]
R = k[NO]2[Cl2]
Slide 9
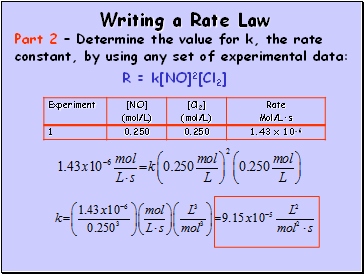
Writing a Rate Law
Part 2 – Determine the value for k, the rate constant, by using any set of experimental data:
R = k[NO]2[Cl2]
Slide 10
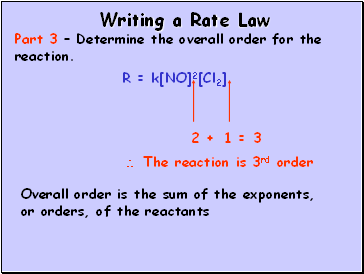
Writing a Rate Law
Part 3 – Determine the overall order for the reaction.
R = k[NO]2[Cl2]
Overall order is the sum of the exponents, or orders, of the reactants
1 2
Contents
- Reaction Rate
- Rate Laws
- Determining Order with Concentration vs. Time data
- Solving an Integrated Rate Law
- Finding the Rate Constant, k
- Rate Laws Summary
Last added presentations
- Static and Kinetic Friction
- Thermal Energy
- Friction
- Newton’s Laws of Motion
- Radiation Safety and Operations
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Heat-Energy on the Move