Atomic TheoryPage
8
8
Plasma
“Superheated Gas”
When atoms are so hot,
they lose ALL
of their
electrons.
Slide 111
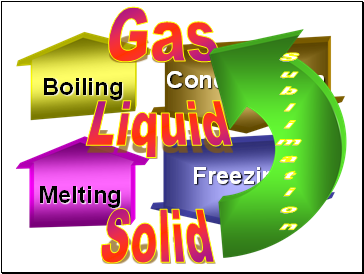
Boiling
Melting
Freezing
Condensation
Solid
Liquid
Gas
Sublimation
Slide 112
Solid
Liquid
Gas
Sublimation
When a solid
turns directly
into a gas.
Dry ice is
solid CO2
Slide 113
Solid
Liquid
Gas
Condensation
When a gas
turns into
into a liquid.
Dry ice is
solid CO2
Slide 114
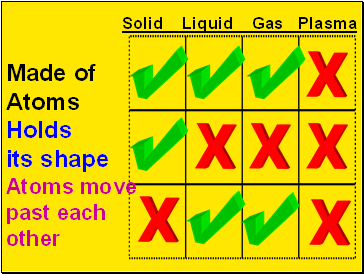
Solid Liquid Gas Plasma
Made of
Atoms
Holds
its shape
Atoms move
past each
other
Slide 115
The solid, liquid, and gaseous states of water differ from each other in
the mass of the individual atoms.
the size of the individual atoms.
the net electrical charge of the individual molecules.
the average speed of movement of the individual molecules.
Slide 116
Fireworks contain different elements in them for displaying different colors. The different colors occur because:
a.the different elements burn at different temperatures.
b.atoms of various elements react with each other differently.
c.atoms of various elements emit light at different frequencies.
d.atoms of different elements have different numbers of protons.
Slide 117
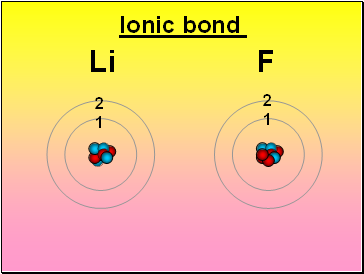
Ionic bond
Li F
2
1
2
1
Slide 118
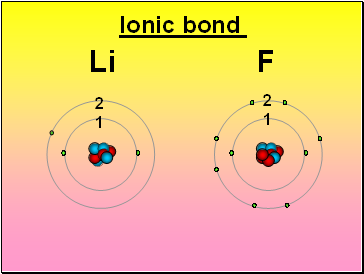
Li F
Ionic bond
2
1
2
1
Slide 119
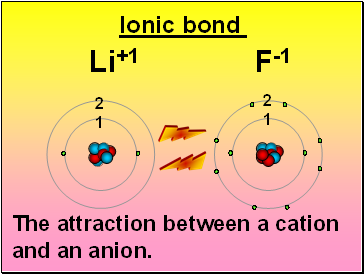
Li+1 F-1
Ionic bond
2
1
2
1
The attraction between a cation and an anion.
Slide 120
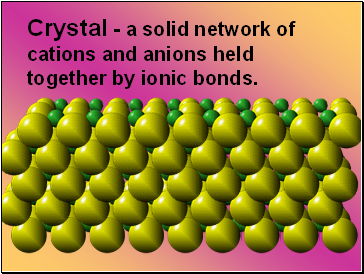
Crystal - a solid network of cations and anions held together by ionic bonds.
Slide 121
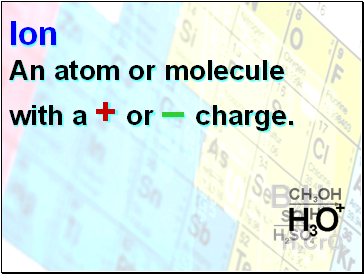
Ion
An atom or molecule with a + or – charge.
Slide 122
Cation
an ion with a positive charge.
Anion
an ion with a Negative charge.
A
I
O
N
+
-
Slide 123
Cations
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Health Physics
- Magnetic field uses sound waves to ignite sun's ring of fire
- Sound
- Direct heat utilization of geothermal energy
- Soil and Plant Nutrition
- Newton’s Law of Gravity
- Radioactivity and Nuclear Reactions