Atomic TheoryPage
10
10
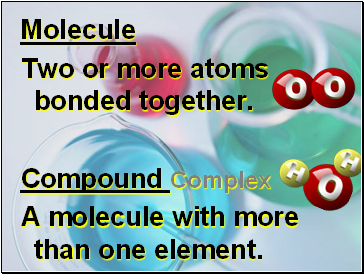
Molecule
Two or more atoms bonded together.
Compound Complex
A molecule with more than one element.
Slide 139
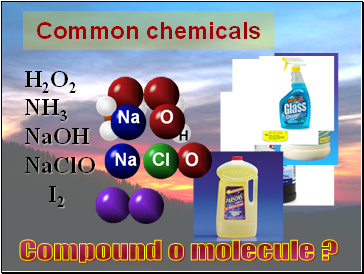
Common chemicals
H2O2
NH3
NaOH
NaClO
I2
Compound o molecule ?
Slide 140
Combustibility
The tendency to react with Oxygen O2 .
Slide 141
H + O2
C + O2
N + O2
O + O2
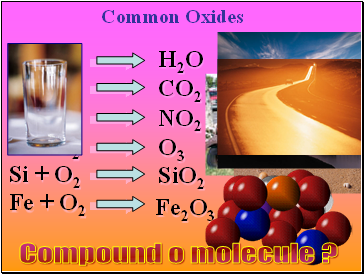
Si + O2
Fe + O2
Common Oxides
H2O
NO2
CO2
O3
SiO2
Fe2O3
Compound o molecule ?
Slide 142
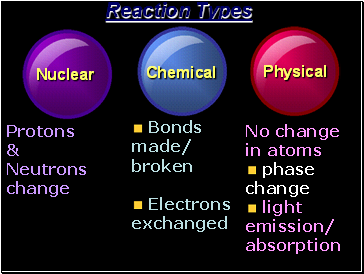
Reaction Types
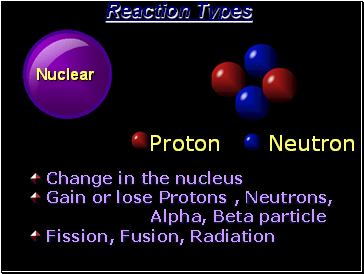
Nuclear
Chemical
Physical
Protons
&
Neutrons
change
Bonds
made/ broken
Electrons exchanged
No change in atoms
phase change
light emission/ absorption
Slide 143
Nuclear
Reaction Types
Change in the
Gain or lose Protons , Neutrons,
Alpha, Beta particle
Fission, Fusion, Radiation
Proton Neutron
nucleus
Slide 144
Slide 145
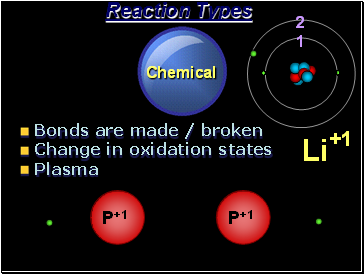
Chemical
Reaction Types
Bonds are made / broken
Change in oxidation states
Plasma
P+1
P+1
2
1
Li
+1
Slide 146
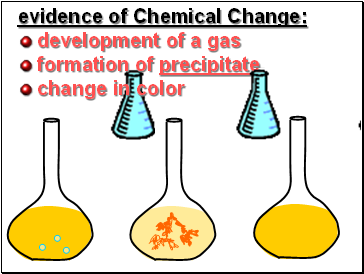
evidence of Chemical Change:
development of a gas
formation of precipitate
change in color
Slide 147
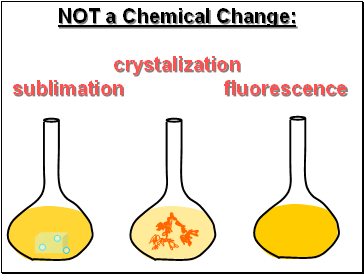
NOT a Chemical Change:
crystalization
sublimation fluorescence
Slide 148
more evidence of
a Chemical Change:
light
fire
http://webmineral.com/help/FlameTest.shtml
Flame Test
Slide 149
Precipitate
formation of insoluble ionic compounds.
Slide 150
You get up in the morning and make toast for breakfast. You notice the color changes from light to dark. Later on that day in science class, your teachers asks for every day examples of physical and chemical changes.
Should you volunteer your toast as an example of a physical or chemical change?
Why?
Slide 151
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- History of Modern Astronomy
- Newton’s law of universal gravitation
- Sound
- Ch 9 Nuclear Radiation
- Health Physics
- Resource Acquisition and Transport in Vascular Plants
- Simulation at NASA for the Space Radiation Effort