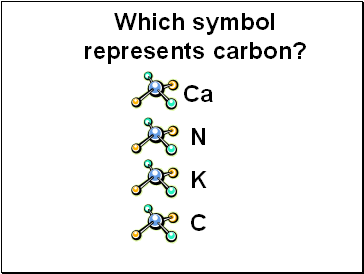Atomic TheoryPage
3
3
Slide 22
Which of the following is a compound?
oxygen
water
nitrogen
air
Slide 23
Evidence of a chemical change would be a
melting popsicle.
spinning top.
spilled bucket of water.
rusting car fender.
Slide 24
Which symbol represents carbon?
Ca
N
K
C
Slide 25
Moisture that collects on the outside of a cold glass results from the process of
evaporation.
condensation.
sublimation.
vaporization.
Slide 26
Slide 27
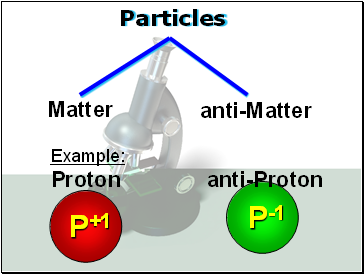
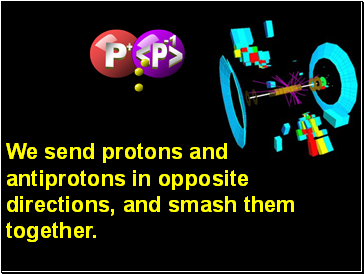
Particles
Matter
P+1
P-1
Example:
Proton anti-Proton
anti-Matter
Slide 28
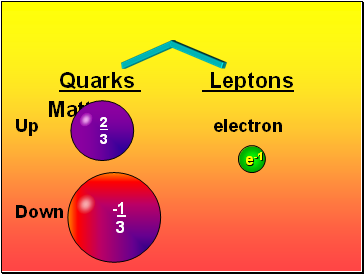
Matter
Quarks Leptons
Up electron
Down
-1
3
2
3
e-1
Slide 29
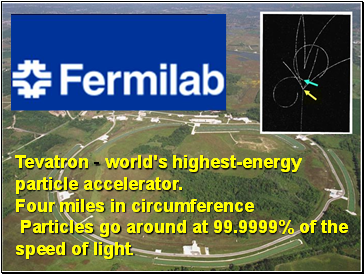
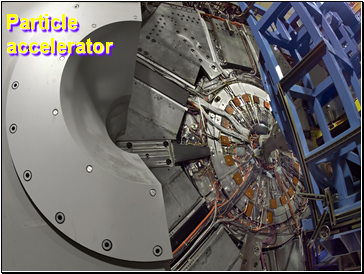
Tevatron - world's highest-energy particle accelerator. Four miles in circumference
Particles go around at 99.9999% of the speed of light.
Slide 30
Slide 31
Particle accelerator
Slide 32
Slide 33
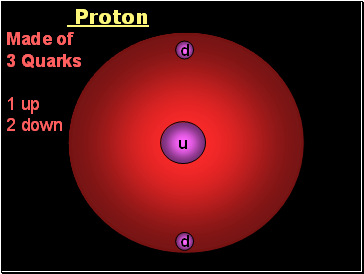
Proton
Made of
3 Quarks
1 up
2 down
u
d
d
Slide 34
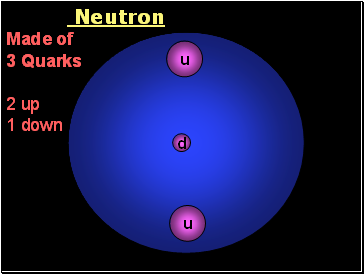
Neutron
Made of
3 Quarks
2 up
1 down
d
u
u
Slide 35
Can we see atoms?
Yes
magnesium atoms (white) above boron atoms (grey) seen by the transmission electron microscope
Slide 36
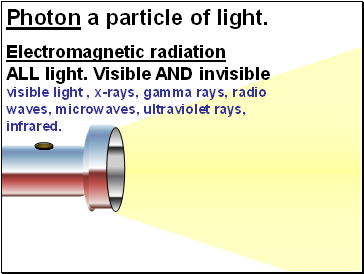
Photon a particle of light.
Electromagnetic radiation
ALL light. Visible AND invisible visible light , x-rays, gamma rays, radio waves, microwaves, ultraviolet rays, infrared.
Slide 37
Photon a particle of light
Laser
Slide 38
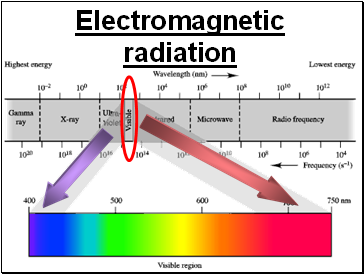
Electromagnetic radiation
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Simulation at NASA for the Space Radiation Effort
- Space Radiation
- Newton’s Laws of Motion
- Radioactivity and Nuclear Reactions
- Newton’s law of universal gravitation
- Upcoming Classes
- Geophysical Concepts, Applications and Limitations