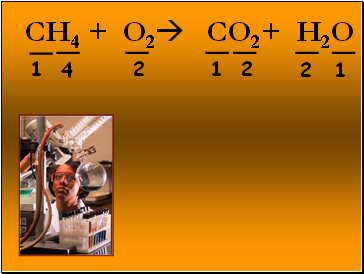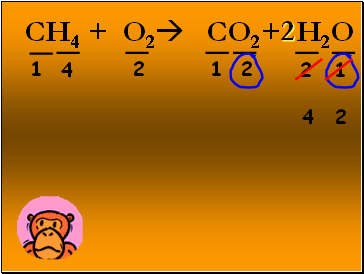Atomic TheoryPage
11
11
Lucy noticed that her coin collection had begun to tarnish. Some of the metal in the coins had begun to change color. The formation of tarnish is most similar to which of the following changes?
shredding a piece of paper into hundreds of tiny strips
dropping a dinner plate on the floor
melting ice cubes in a glass of juice
burning a piece of paper to ashes in a fireplace
Slide 152
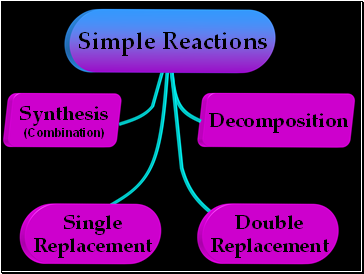
Decomposition
Simple Reactions
Synthesis
(Combination)
Single
Replacement
Double
Replacement
Slide 153
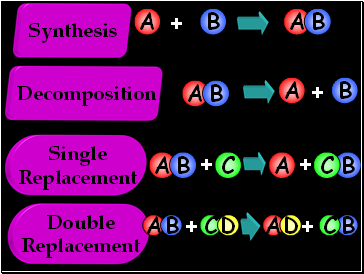
Decomposition
Synthesis
Single
Replacement
Double
Replacement
A
B
A
B
+
A
B
A
B
+
A
B
A
B
+
C
+
C
A
B
A
B
+
C
+
C
D
D
Slide 154
Decomposition
Synthesis
Single
Replacement
Double
Replacement
A
B
A
B
+
A
B
A
B
+
A
B
A
B
+
C
+
C
A
B
A
B
+
C
+
C
D
D
Slide 155
+
+
+
+
+
+
Synthesis
Decomposition
Single
Replacement
Double
Replacement
Slide 156
A displacement reaction:
metallic copper with silver nitrate
Cu + Ag NO3
Ag + Cu(NO3)2
Slide 157
Balancing equations
Slide 158
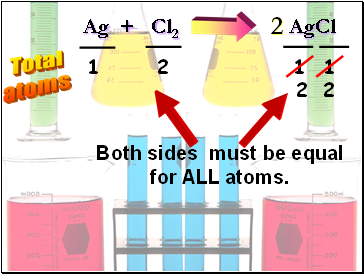
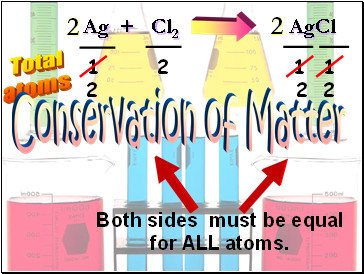
Ag + Cl2 AgCl
Total
atoms
1
2
1
1
Both sides must be equal
for ALL atoms.
2
2
2
Slide 159
Ag + Cl2 AgCl
Total
atoms
1
2
1
1
Both sides must be equal
for ALL atoms.
2
2
2
2
2
Conservation of Matter
Slide 160
CH4 + O2 CO2+ H2O
1
2
1
2
4
2
1
Slide 161
CH4 + O2 CO2+ H2O
1
2
1
2
4
2
1
2
2
4
Slide 162
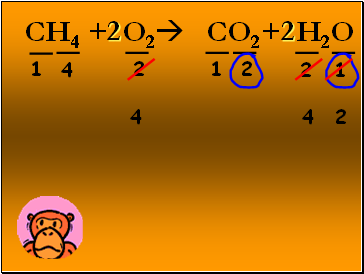
CH4 + O2 CO2+ H2O
1
2
1
2
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Heat-Energy on the Move
- Newton’s law of universal gravitation
- Simulation at NASA for the Space Radiation Effort
- Magnetic field uses sound waves to ignite sun's ring of fire
- Motion
- Radiation Safety and Operations