Atomic TheoryPage
4
4
Slide 39
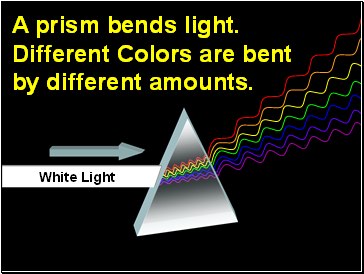
A prism bends light.
Different Colors are bent
by different amounts.
White Light
Slide 40
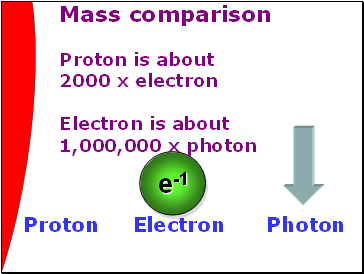
e-1
.
Proton Electron Photon
Mass comparison
Proton is about
2000 x electron
Electron is about
1,000,000 x photon
Slide 41
Matter
DO everything be made of matter ?
What are the building blocks of matter ?
How many elements are there?
What B da opposite of a mixture ?
Experiment (candle/hot air balloon)
Slide 42
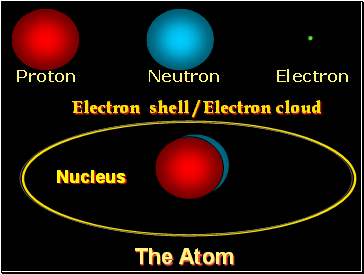
Proton Neutron Electron
The Atom
Nucleus
Electron shell / Electron cloud
Slide 43
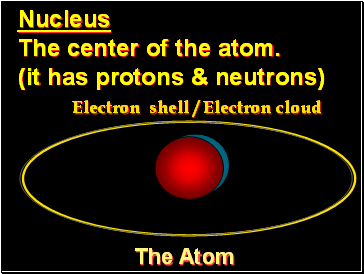
The Atom
Nucleus
The center of the atom.
(it has protons & neutrons)
Electron shell / Electron cloud
Slide 44
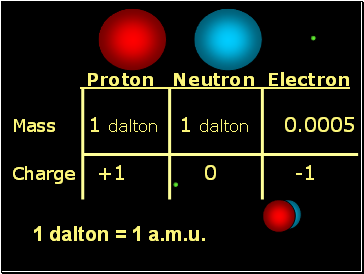
Proton Neutron Electron
Mass 1 dalton 1 dalton 0.0005
Charge +1 0 -1
1 dalton = 1 a.m.u.
Slide 45
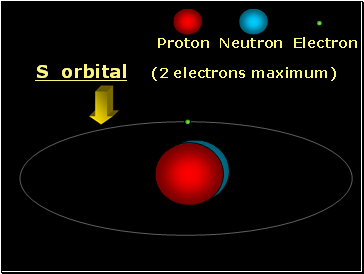
Proton Neutron Electron
S orbital (2 electrons maximum)
Slide 46
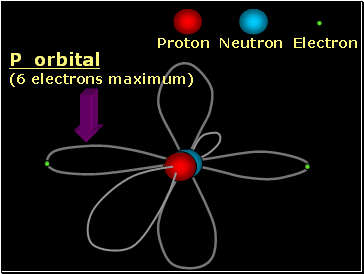
Proton Neutron Electron
P orbital
(6 electrons maximum)
Slide 47
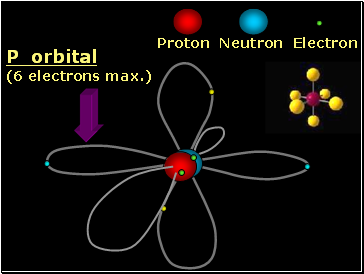
Proton Neutron Electron
P orbital
(6 electrons max.)
Slide 48
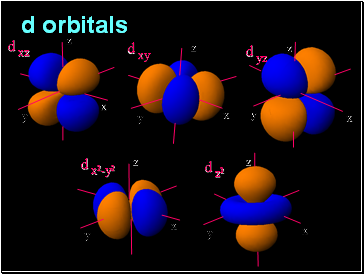
d orbitals
Slide 49
Proton Neutron Electron
S orbital P orbital
Slide 50
1st Shell of electrons
S orbital
Slide 51
2nd Shell of electrons
S orbital P orbital
Slide 52
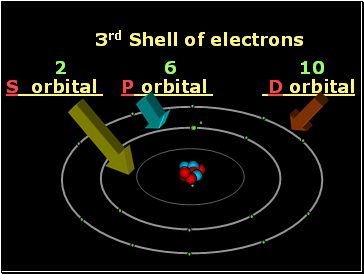
3rd Shell of electrons
S orbital P orbital D orbital
2 6 10
Slide 53
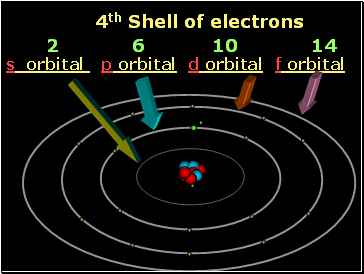
4th Shell of electrons
s orbital p orbital d orbital f orbital
2 6 10 14
Slide 54
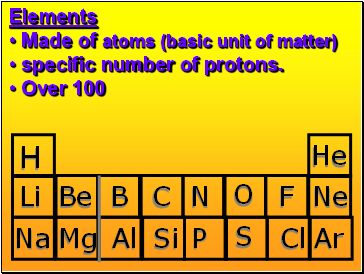
Element
Atom(s) having a specific number of Protons.
Slide 55
Contents
- Ionic bond
- Ion
- Cations
- Non-Metals
- Metalloids
- States of Matter
- Neon
- Valence Electrons
- Isotopes
- Electron Shells
- Atomic Number
- Hydrogen
- Helium
- Lithium
- Beryllium
- Boron
- Carbon
- Nitrogen
- Oxygen
- Fluorine
- Element
- Which of the following is a compound?
- Which symbol represents carbon?
- Particle accelerator
- Proton
- Neutron
- Electromagnetic radiation
- Which formulas represent compounds?
- Which is an example of a chemical change?
- Which statement is correct concerning the mass of a ball of clay?
- Mary wants to find the density of a small stone. Which tools will she need?
- Forces
- Particles
- Which pair of elements is MOST similar?
- Anions
- Solution
- Covalent bond
- Common chemicals
- Combustibility
- Reaction Types
- Precipitate
- Balancing equations
- Distilled water
Last added presentations
- Friction
- Solar Thermal Energy
- Radiation Safety and Operations
- Thermal Energy
- Gravitation
- Ch 9 Nuclear Radiation
- Upcoming Classes