Atomic StructurePage
3
3
Slide 12
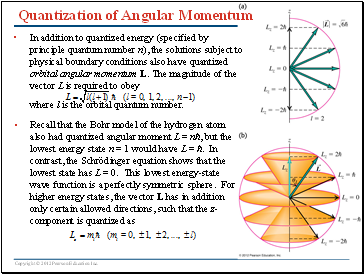
Quantization of Angular Momentum
In addition to quantized energy (specified by principle quantum number n), the solutions subject to physical boundary conditions also have quantized orbital angular momentum L. The magnitude of the vector L is required to obey
where l is the orbital quantum number.
Recall that the Bohr model of the hydrogen atom also had quantized angular moment L = nħ, but the lowest energy state n = 1 would have L = ħ. In contrast, the Schrödinger equation shows that the lowest state has L = 0. This lowest energy-state wave function is a perfectly symmetric sphere. For higher energy states, the vector L has in addition only certain allowed directions, such that the z-component is quantized as
Slide 13
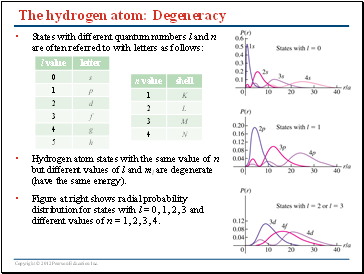
The hydrogen atom: Degeneracy
States with different quantum numbers l and n are often referred to with letters as follows:
Hydrogen atom states with the same value of n but different values of l and ml are degenerate (have the same energy).
Figure at right shows radial probability distribution for states with l = 0, 1, 2, 3 and different values of n = 1, 2, 3, 4.
Slide 14
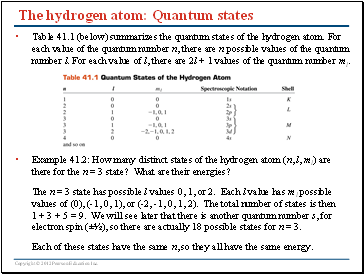
The hydrogen atom: Quantum states
Table 41.1 (below) summarizes the quantum states of the hydrogen atom. For each value of the quantum number n, there are n possible values of the quantum number l. For each value of l, there are 2l + 1 values of the quantum number ml.
Example 41.2: How many distinct states of the hydrogen atom (n, l, ml) are there for the n = 3 state? What are their energies?
The n = 3 state has possible l values 0, 1, or 2. Each l value has ml possible values of (0), (-1, 0, 1), or (-2, -1, 0, 1, 2). The total number of states is then 1 + 3 + 5 = 9. We will see later that there is another quantum number s, for electron spin (±½), so there are actually 18 possible states for n = 3.
Each of these states have the same n, so they all have the same energy.
Slide 15
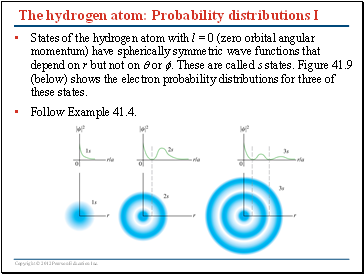
The hydrogen atom: Probability distributions I
States of the hydrogen atom with l = 0 (zero orbital angular momentum) have spherically symmetric wave functions that depend on r but not on or . These are called s states. Figure 41.9 (below) shows the electron probability distributions for three of these states.
Follow Example 41.4.
Slide 16
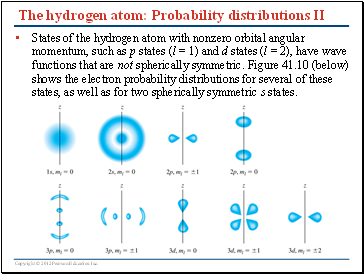
The hydrogen atom: Probability distributions II
States of the hydrogen atom with nonzero orbital angular momentum, such as p states (l = 1) and d states (l = 2), have wave functions that are not spherically symmetric. Figure 41.10 (below) shows the electron probability distributions for several of these states, as well as for two spherically symmetric s states.
Contents
- The Schrödinger equation in 3-D
- Particle in a three-dimensional box
- Energy Degeneracy
- The hydrogen atom: Quantum numbers
- Description of solution
- Quantization of Angular Momentum
- The hydrogen atom: Degeneracy
- The hydrogen atom: Quantum states
- The hydrogen atom: Probability distributions I
- Magnetic moments and the Zeeman effect
- The Zeeman effect and selection rules
- The anomalous Zeeman effect and electron spin
- Electron spin and the Stern-Gerlach experiment
- Quantum states and the Pauli exclusion principle
- A multielectron atom
- Ground-state electron configurations
- Screening in multielectron atoms
- X-ray spectroscopy
Last added presentations
- Sound
- Motion
- Newton's Laws
- Buoyancy
- Newton's laws of motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s Laws of Motion