Atomic StructurePage
4
4
Slide 17
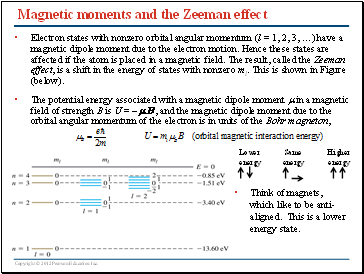
Magnetic moments and the Zeeman effect
Electron states with nonzero orbital angular momentum (l = 1, 2, 3, …) have a magnetic dipole moment due to the electron motion. Hence these states are affected if the atom is placed in a magnetic field. The result, called the Zeeman effect, is a shift in the energy of states with nonzero ml. This is shown in Figure (below).
The potential energy associated with a magnetic dipole moment m in a magnetic field of strength B is U = -mB , and the magnetic dipole moment due to the orbital angular momentum of the electron is in units of the Bohr magneton,
Think of magnets, which like to be anti-aligned. This is a lower energy state.
Slide 18
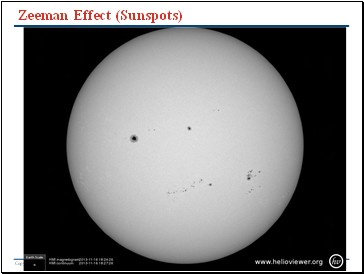
Zeeman Effect (Sunspots)
Slide 19
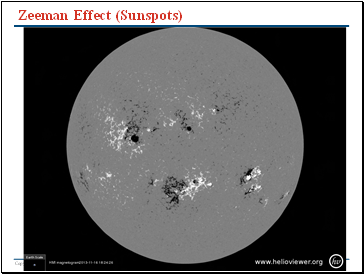
Zeeman Effect (Sunspots)
Slide 20
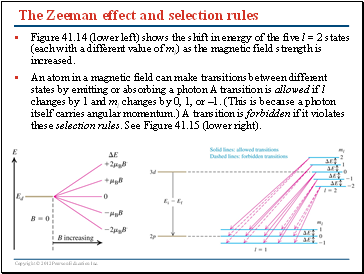
The Zeeman effect and selection rules
Figure 41.14 (lower left) shows the shift in energy of the five l = 2 states (each with a different value of ml) as the magnetic field strength is increased.
An atom in a magnetic field can make transitions between different states by emitting or absorbing a photon A transition is allowed if l changes by 1 and ml changes by 0, 1, or –1. (This is because a photon itself carries angular momentum.) A transition is forbidden if it violates these selection rules. See Figure 41.15 (lower right).
Slide 21
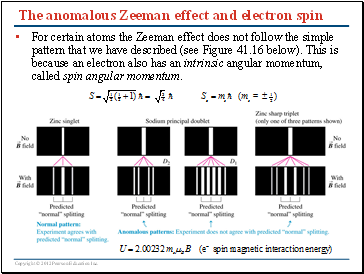
The anomalous Zeeman effect and electron spin
For certain atoms the Zeeman effect does not follow the simple pattern that we have described (see Figure 41.16 below). This is because an electron also has an intrinsic angular momentum, called spin angular momentum.
Slide 22
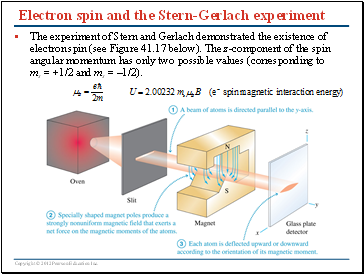
Electron spin and the Stern-Gerlach experiment
The experiment of Stern and Gerlach demonstrated the existence of electron spin (see Figure 41.17 below). The z-component of the spin angular momentum has only two possible values (corresponding to ms = +1/2 and ms = –1/2).
Slide 23
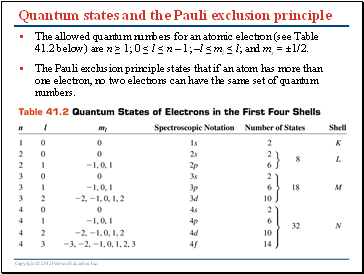
Quantum states and the Pauli exclusion principle
The allowed quantum numbers for an atomic electron (see Table 41.2 below) are n ≥ 1; 0 ≤ l ≤ n – 1; –l ≤ ml ≤ l; and ms = ±1/2.
The Pauli exclusion principle states that if an atom has more than one electron, no two electrons can have the same set of quantum numbers.
Slide 24
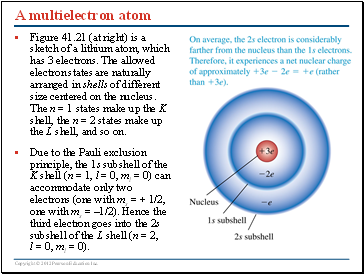
A multielectron atom
Contents
- The Schrödinger equation in 3-D
- Particle in a three-dimensional box
- Energy Degeneracy
- The hydrogen atom: Quantum numbers
- Description of solution
- Quantization of Angular Momentum
- The hydrogen atom: Degeneracy
- The hydrogen atom: Quantum states
- The hydrogen atom: Probability distributions I
- Magnetic moments and the Zeeman effect
- The Zeeman effect and selection rules
- The anomalous Zeeman effect and electron spin
- Electron spin and the Stern-Gerlach experiment
- Quantum states and the Pauli exclusion principle
- A multielectron atom
- Ground-state electron configurations
- Screening in multielectron atoms
- X-ray spectroscopy
Last added presentations
- Practical Applications of Solar Energy
- Waves & Sound
- Newton’s Law of Gravity
- Thermal Energy
- Solar Thermal Energy
- Sensory and Motor Mechanisms
- Sound