The Chemistry of Acids and BasesPage
1
1
Slide 1
The Chemistry of Acids and Bases
Chemistry I – Chapter 19
Chemistry I HD – Chapter 16
ICP – Chapter 23
SAVE PAPER AND INK!!! When you print out the notes on PowerPoint, print "Handouts" instead of "Slides" in the print setup. Also, turn off the backgrounds (Tools>Options>Print>UNcheck "Background Printing")!
Slide 2
Acid and Bases
Slide 3
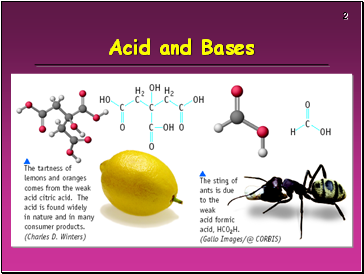
Acid and Bases
Slide 4
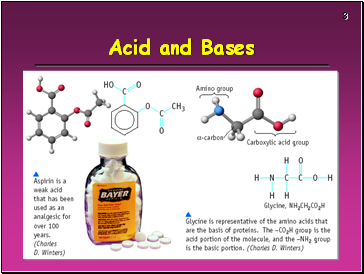
Acid and Bases
Slide 5
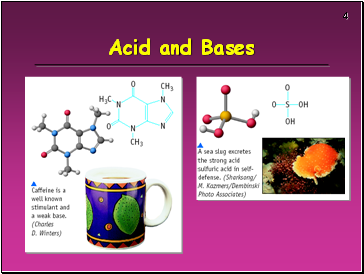
Acids
Have a sour taste. Vinegar is a solution of acetic acid. Citrus
fruits contain citric acid.
React with certain metals to produce hydrogen gas.
React with carbonates and bicarbonates to produce carbon
dioxide gas
Have a bitter taste.
Feel slippery. Many soaps contain bases.
Bases
Slide 6
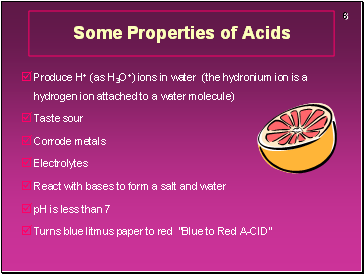
Some Properties of Acids
Produce H+ (as H3O+) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule)
Taste sour
Corrode metals
Electrolytes
React with bases to form a salt and water
pH is less than 7
Turns blue litmus paper to red “Blue to Red A-CID”
Slide 7
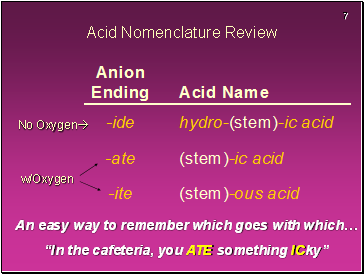
Acid Nomenclature Review
No Oxygen
w/Oxygen
An easy way to remember which goes with which…
“In the cafeteria, you ATE something ICky”
Slide 8
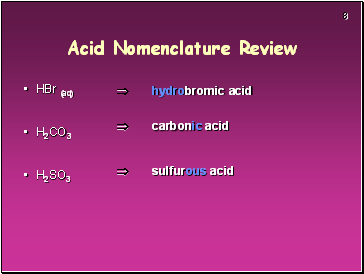
Acid Nomenclature Review
HBr (aq)
H2CO3
H2SO3
hydrobromic acid
carbonic acid
sulfurous acid
Slide 9
Name ‘Em!
HI (aq)
HCl (aq)
H2SO3
HNO3
HIO4
Slide 10
Some Properties of Bases
Produce OH- ions in water
Taste bitter, chalky
Are electrolytes
Feel soapy, slippery
React with acids to form salts and water
pH greater than 7
Turns red litmus paper to blue “Basic Blue”
Slide 11
Some Common Bases
NaOH sodium hydroxide lye
KOH potassium hydroxide liquid soap
Ba(OH)2 barium hydroxide stabilizer for plastics
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Gravitation
- Motion
- Solar Energy
- Health Physics
- Upcoming Classes
- Sound
- Newton’s law of universal gravitation