The Chemistry of Acids and BasesPage
3
3
Slide 24
pH of Common Substances
Slide 25
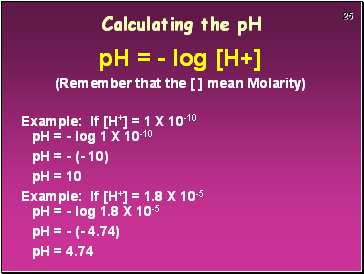
Calculating the pH
pH = - log [H+]
(Remember that the [ ] mean Molarity)
Example: If [H+] = 1 X 10-10 pH = - log 1 X 10-10
pH = - (- 10)
pH = 10
Example: If [H+] = 1.8 X 10-5 pH = - log 1.8 X 10-5
pH = - (- 4.74)
pH = 4.74
Slide 26
Try These!
Find the pH of these:
1) A 0.15 M solution of Hydrochloric acid
2) A 3.00 X 10-7 M solution of Nitric acid
Slide 27
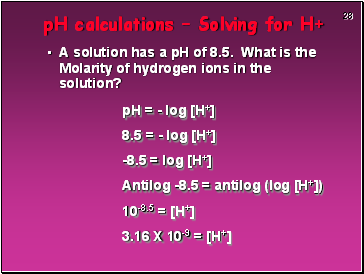
pH calculations – Solving for H+
If the pH of Coke is 3.12, [H+] = ???
Because pH = - log [H+] then
- pH = log [H+]
Take antilog (10x) of both sides and get
10-pH = [H+]
[H+] = 10-3.12 = 7.6 x 10-4 M
*** to find antilog on your calculator, look for “Shift” or “2nd function” and then the log button
Slide 28
pH calculations – Solving for H+
A solution has a pH of 8.5. What is the Molarity of hydrogen ions in the solution?
pH = - log [H+]
8.5 = - log [H+]
-8.5 = log [H+]
Antilog -8.5 = antilog (log [H+])
10-8.5 = [H+]
3.16 X 10-9 = [H+]
Slide 29
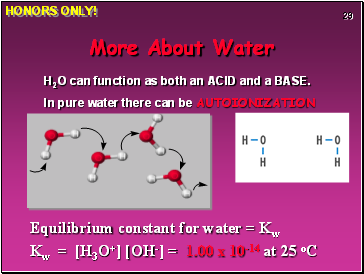
More About Water
H2O can function as both an ACID and a BASE.
In pure water there can be AUTOIONIZATION
Equilibrium constant for water = Kw
Kw = [H3O+] [OH-] = 1.00 x 10-14 at 25 oC
HONORS ONLY!
Slide 30
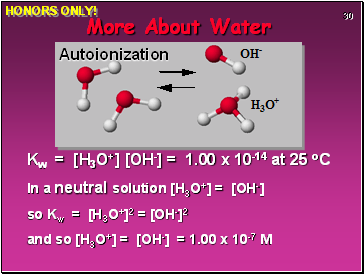
More About Water
Kw = [H3O+] [OH-] = 1.00 x 10-14 at 25 oC
In a neutral solution [H3O+] = [OH-]
so Kw = [H3O+]2 = [OH-]2
and so [H3O+] = [OH-] = 1.00 x 10-7 M
Autoionization
HONORS ONLY!
Slide 31
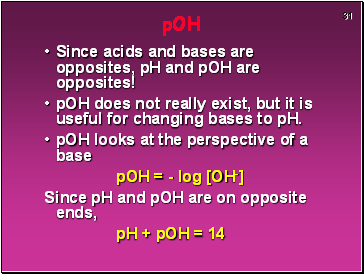
pOH
Since acids and bases are opposites, pH and pOH are opposites!
pOH does not really exist, but it is useful for changing bases to pH.
pOH looks at the perspective of a base
pOH = - log [OH-]
Since pH and pOH are on opposite ends,
pH + pOH = 14
Slide 32
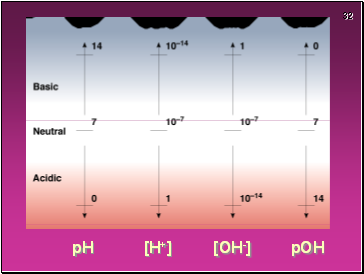
pH
[H+]
[OH-]
pOH
Slide 33
[H3O+], [OH-] and pH
What is the pH of the 0.0010 M NaOH solution?
[OH-] = 0.0010 (or 1.0 X 10-3 M)
pOH = - log 0.0010
pOH = 3
pH = 14 – 3 = 11
OR Kw = [H3O+] [OH-]
[H3O+] = 1.0 x 10-11 M
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Practical Applications of Solar Energy
- Madame Marie Curie
- Space Radiation
- Buoyancy
- History of Modern Astronomy
- Newton's laws of motion
- Static and Kinetic Friction




![[H3O+], [OH-] and pH [H3O+], [OH-] and pH](images/referats/1143/image033.png)