The Chemistry of Acids and BasesPage
7
7
You should only use a small portion of the paper. You can use one piece of paper for several tests.
Slide 61
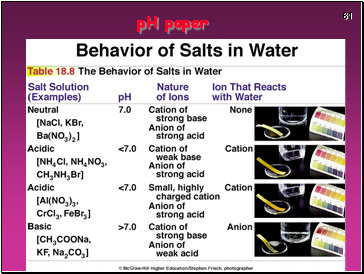
pH paper
Slide 62
pH meter
Tests the voltage of the electrolyte
Converts the voltage to pH
Very cheap, accurate
Must be calibrated with a buffer solution
Slide 63
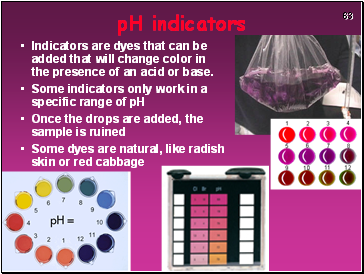
pH indicators
Indicators are dyes that can be added that will change color in the presence of an acid or base.
Some indicators only work in a specific range of pH
Once the drops are added, the sample is ruined
Some dyes are natural, like radish skin or red cabbage
Slide 64
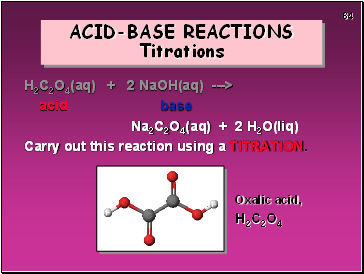
ACID-BASE REACTIONS Titrations
H2C2O4(aq) + 2 NaOH(aq) --->
acid base
Na2C2O4(aq) + 2 H2O(liq)
Carry out this reaction using a TITRATION.
Slide 65
Setup for titrating an acid with a base
Slide 66
Titration
1. Add solution from the buret.
2. Reagent (base) reacts with compound (acid) in solution in the flask.
Indicator shows when exact stoichiometric reaction has occurred. (Acid = Base)
This is called NEUTRALIZATION.
Slide 67
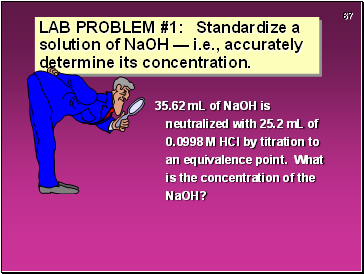
35.62 mL of NaOH is neutralized with 25.2 mL of 0.0998 M HCl by titration to an equivalence point. What is the concentration of the NaOH?
LAB PROBLEM #1: Standardize a solution of NaOH — i.e., accurately determine its concentration.
Slide 68
PROBLEM: You have 50.0 mL of 3.0 M NaOH and you want 0.50 M NaOH. What do you do?
Add water to the 3.0 M solution to lower its concentration to 0.50 M
Dilute the solution!
Slide 69
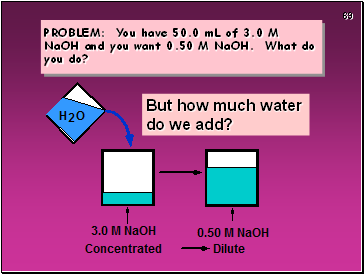
PROBLEM: You have 50.0 mL of 3.0 M NaOH and you want 0.50 M NaOH. What do you do?
But how much water
do we add?
Slide 70
PROBLEM: You have 50.0 mL of 3.0 M NaOH and you want 0.50 M NaOH. What do you do?
How much water is added?
The important point is that --->
Slide 71
PROBLEM: You have 50.0 mL of 3.0 M NaOH and you want 0.50 M NaOH. What do you do?
Amount of NaOH in original solution =
M • V =
(3.0 mol/L)(0.050 L) = 0.5 M NaOH X V
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Radiation
- Mechanics Lecture
- Newton’s Law of Gravity
- Simulation at NASA for the Space Radiation Effort
- Newton’s law of universal gravitation
- Direct heat utilization of geothermal energy
- Sound