The Chemistry of Acids and BasesPage
5
5
K gives the ratio of ions (split up) to molecules (don’t split up)
HONORS ONLY!
Slide 44
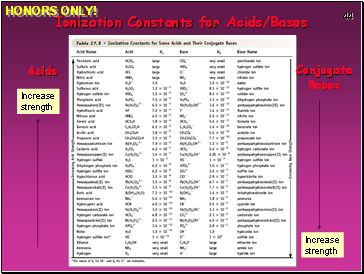
Ionization Constants for Acids/Bases
Acids
Conjugate
Bases
Increase strength
Increase strength
HONORS ONLY!
Slide 45
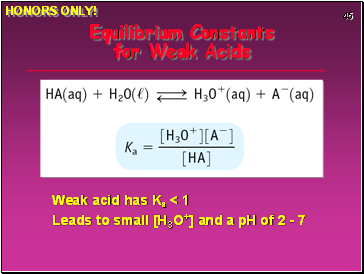
Equilibrium Constants for Weak Acids
Weak acid has Ka < 1
Leads to small [H3O+] and a pH of 2 - 7
HONORS ONLY!
Slide 46
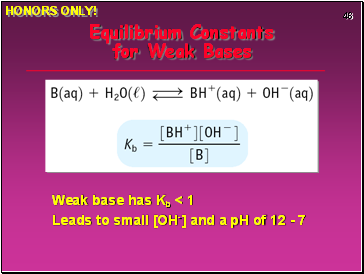
Equilibrium Constants for Weak Bases
Weak base has Kb < 1
Leads to small [OH-] and a pH of 12 - 7
HONORS ONLY!
Slide 47
Relation of Ka, Kb, [H3O+] and pH
HONORS ONLY!
Slide 48
Equilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H3O+, OAc-, and the pH.
Step 1. Define equilibrium concs. in ICE table.
[HOAc] [H3O+] [OAc-]
initial
change
equilib
1.00 0 0
-x +x +x
1.00-x x x
HONORS ONLY!
Slide 49
Equilibria Involving A Weak Acid
Step 2. Write Ka expression
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H3O+, OAc-, and the pH.
This is a quadratic. Solve using quadratic formula.
or you can make an approximation if x is very small! (Rule of thumb: 10-5 or smaller is ok)
HONORS ONLY!
Slide 50
Equilibria Involving A Weak Acid
Step 3. Solve Ka expression
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H3O+, OAc-, and the pH.
First assume x is very small because Ka is so small.
Now we can more easily solve this approximate expression.
HONORS ONLY!
Slide 51
Approximating
If K is really small, the equilibrium concentrations will be nearly the same as the initial concentrations.
Example: 0.20 – x is just about 0.20 if x is really small.
If the K is 10-5 or smaller (10-6, 10-7, etc.), you should approximate. Otherwise, you have to use the quadratic.
Slide 52
Equilibria Involving A Weak Acid
Step 3. Solve Ka approximate expression
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H3O+, OAc-, and the pH.
x = [H3O+] = [OAc-] = 4.2 x 10-3 M
pH = - log [H3O+] = -log (4.2 x 10-3) = 2.37
HONORS ONLY!
Slide 53
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Soil and Plant Nutrition
- Mechanics Lecture
- Ch 9 Nuclear Radiation
- Radiation Safety and Operations
- Newton’s laws of motion
- Geophysical Concepts, Applications and Limitations
- Direct heat utilization of geothermal energy

![Relation of Ka, Kb, [H3O+] and pH Relation of Ka, Kb, [H3O+] and pH](images/referats/1143/image047.png)





