The Chemistry of Acids and BasesPage
8
8
Amount of NaOH in final solution must also = 0.15 mol NaOH
Volume of final solution =
(0.15 mol NaOH) / (0.50 M) = 0.30 L
or 300 mL
Slide 72
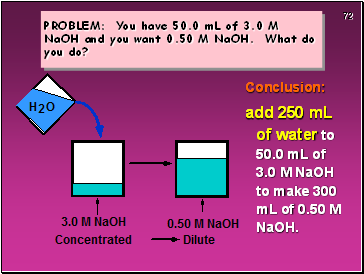
PROBLEM: You have 50.0 mL of 3.0 M NaOH and you want 0.50 M NaOH. What do you do?
Conclusion:
add 250 mL of water to 50.0 mL of 3.0 M NaOH to make 300 mL of 0.50 M NaOH.
Slide 73
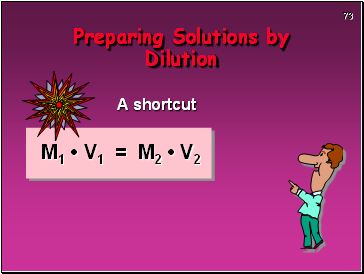
A shortcut
M1 • V1 = M2 • V2
Preparing Solutions by Dilution
Slide 74
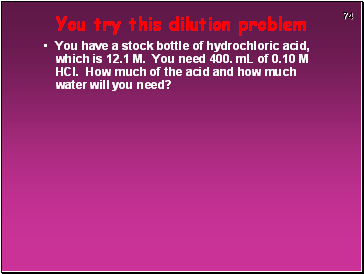
You try this dilution problem
You have a stock bottle of hydrochloric acid, which is 12.1 M. You need 400. mL of 0.10 M HCl. How much of the acid and how much water will you need?
Contents
- Acid and Bases
- Some Properties of Acids
- Acid Nomenclature Review
- Some Properties of Bases
- Some Common Bases
- Acid/Base definitions
- Lewis Acids & Bases
- Titration
Last added presentations
- Newton's laws of motion
- Sound
- Magnetic field uses sound waves to ignite sun's ring of fire
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Direct heat utilization of geothermal energy
- Mechanics Lecture
- Space Radiation
© 2010-2026 powerpoint presentations