Covalent Bonding (Molecules)Page
2
2
C
H
H
H
Cl
Add up available valence electrons:
C = 4, H = (3)(1), Cl = 7 Total = 14
Join peripheral atoms
to the central atom with electron pairs.
Complete octets on
atoms other than hydrogen with remaining electrons
Make carbon the central atom (it wants the most bonds, 4)
Slide 10
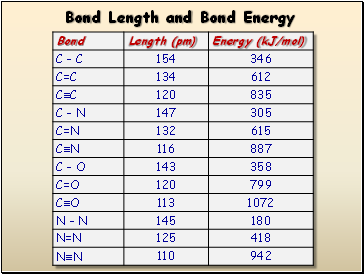
Bond Length and Bond Energy
Slide 11
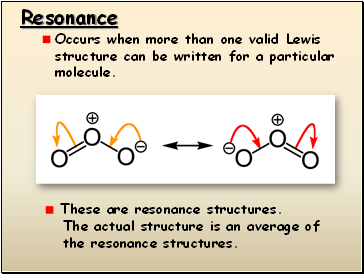
Resonance
Occurs when more than one valid Lewis structure can be written for a particular molecule.
These are resonance structures.
The actual structure is an average of
the resonance structures.
Slide 12
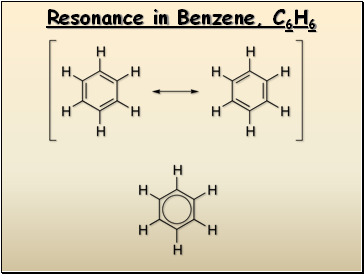
Resonance in Benzene, C6H6
Go to page:
1 2
1 2
Contents
- Covalent Bonding
- CA Standards
- The Octet Rule and Covalent Compounds
- The Octet Rule: The Diatomic Fluorine Molecule
- The Octet Rule: The Diatomic Oxygen Molecule
- The Octet Rule: The Diatomic Nitrogen Molecule
- Lewis Structures
- The HONC Rule
- Completing a Lewis Structure -CH3Cl
- Bond Length and Bond Energy
- Resonance
- Resonance in Benzene, C6H6
Last added presentations
- Direct heat utilization of geothermal energy
- Gravitation
- Newton’s third law of motion
- Newton’s laws of motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- Buoyancy
- History of Modern Astronomy
© 2010-2026 powerpoint presentations