Atomic Structure and Periodic TrendsPage
8
8
Make multi electron atom look like a one electron atom
Assumes every electron to be on its own experiencing an effective nuclear charge, Zeff
Then the orbitals for the electrons take the form of those in hydrogen but their energies and sizes are modified by simply using an effective nuclear charge
One electron atom
2+
-
-
-
-
Zeff
Two-electron atom
Orbital Approximation
Slide 66
The Concept of Electron Spin
The solution of the Schrödinger equation above accounts very well for the structure of the H-atom spectrum and, as we will see, for other atoms too.
However under very high resolution “fine structure” is observed in the transitions which is not explained using this approach.
Dirac extended wave mechanics to include Special relativity and an extra coordinate - time.
Solution of the Dirac equation yields a new intrinsic angular momentum -
Slide 67
The Electron Spin
An electron has
The projection of this spin is also quantized (by analogy with orbital angular momentum; l and ml) such that the spin projection quantum number, ms=½, (spin up or a) and ms=-½, (spin down or b)
This is the final theoretical plank behind the structure of the periodic table.
Important
Slide 68
Pauli Exclusion Principle
(as all electrons necessarily have the same s=1/2)
Hence, no individual orbital may be occupied by more than 2 electrons
Electrons occupying the same orbital must be “paired up”.
Important
no two electrons in the same atom can have the same 4 quantum numbers n, l, ml, ms
Slide 69
The Pauli Principle and Symmetry
The Pauli Exclusion Principle applies to any
pair of identical (spin quantum
number s = half integer) – protons, electrons,
neutrons have s = ½ and are fermions.
BUT, any number of (s = integer)
can occupy the same orbital - 12C (s = 0) and
the photon (s = 1)are bosons
Slide 70
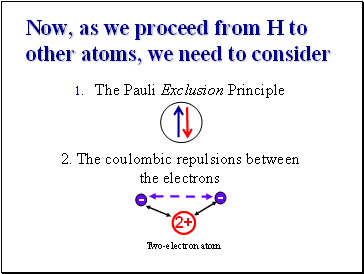
Now, as we proceed from H to other atoms, we need to consider
The Pauli Exclusion Principle
2. The coulombic repulsions between the electrons
2+
-
-
Two-electron atom
Slide 71
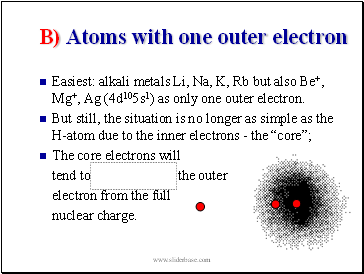
B) Atoms with one outer electron
Easiest: alkali metals Li, Na, K, Rb but also Be+, Mg+, Ag (4d105s1) as only one outer electron.
But still, the situation is no longer as simple as the H-atom due to the inner electrons - the “core”;
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Practical Applications of Solar Energy
- Ch 9 Nuclear Radiation
- Sound
- Friction
- Gravitation
- Direct heat utilization of geothermal energy
- Madame Marie Curie