Atomic Structure and Periodic TrendsPage
13
13
Ti3+
1s22s22p63s23p63d1
Slide 109
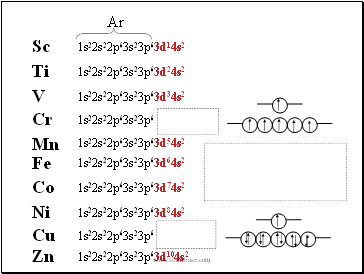
Sc
Ti
1s22s22p63s23p63d14s2
1s22s22p63s23p63d24s2
V
1s22s22p63s23p63d34s2
Cr
1s22s22p63s23p6
Mn
1s22s22p63s23p63d54s2
Fe
Co
1s22s22p63s23p63d64s2
1s22s22p63s23p63d74s2
Ni
1s22s22p63s23p63d84s2
Cu
1s22s22p63s23p6
Zn
1s22s22p63s23p63d104s2
Ar
Slide 110
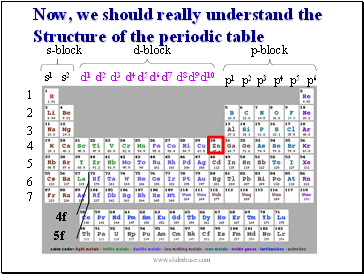
Now, we should really understand the Structure of the periodic table
1
2
3
4
5
6
7
s1
s2
p1
p2
p3
p4
p5
p6
s-block
p-block
d1
d2
d3
d4
d5
d6
d7
d8
d9
d10
d-block
4f
5f
Slide 111
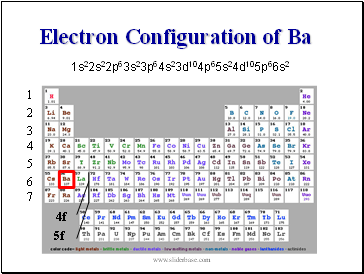
Electron Configuration of Ba
1s22s22p63s23p64s23d104p65s24d105p66s2
1
2
3
4
5
6
7
4f
5f
Slide 112
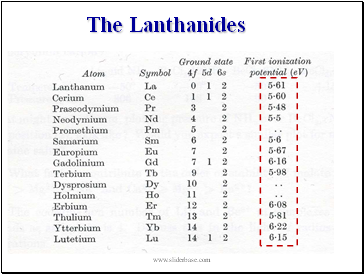
The Lanthanides
Slide 113
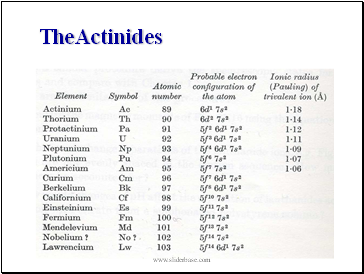
TheActinides
Slide 114
3d
4d
5d
6d
4f
5f
Inner Transition Metals
(f1-14)
Transition Metals
(d1-10)
Noble
Gases
Halogens
(p5)
2p
3p
4p
5p
6p
7p
1s
2s
3s
4s
5s
6s
7s
Alkaline
Earth Metals (s2)
Alkali
Metals (s1)
Slide 115
3d
4d
5d
6d
2p
3p
4p
5p
6p
7p
1s
2s
3s
4s
5s
6s
7s
4f
5f
The Periodic Table of the Elements
Slide 116
Slide 117
Periodic Trends
Slide 118
Periodic Trends
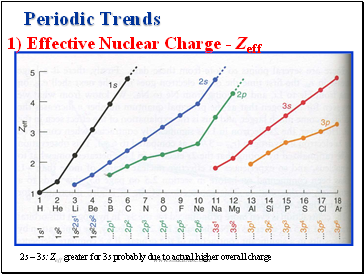
1) Effective Nuclear Charge - Zeff
2s – 3s: Zeff greater for 3s probably due to actual higher overall charge
Slide 119
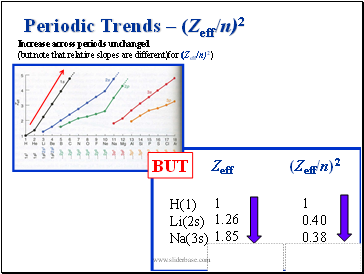
Periodic Trends – (Zeff/n)2
Increase across periods unchanged
(but note that relative slopes are different)for (Zeff/n)2)
BUT
(Zeff/n)2
H(1)
Li(2s)
Na(3s)
Zeff
1
1.26
1.85
1
0.40
0.38
Slide 120
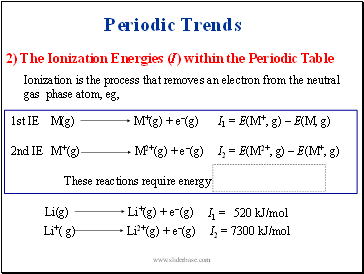
M(g)
M+(g) + e-(g)
Ionization is the process that removes an electron from the neutral gas phase atom, eg,
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Madame Marie Curie
- Newton's laws of motion
- Resource Acquisition and Transport in Vascular Plants
- Mechanics Lecture
- Radioactivity and Nuclear Reactions
- Magnetic field uses sound waves to ignite sun's ring of fire
- The Effects of Radiation on Living Things