Atomic Structure and Periodic TrendsPage
10
10
Slide 81
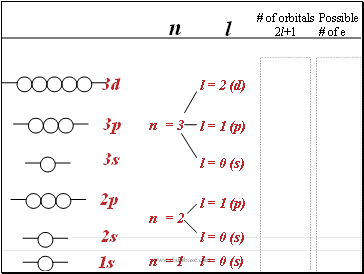
n
l
Possible
# of e
n = 2
2p
n = 3
3s
3p
3d
l = 1 (p)
# of orbitals
2l+1
2s
Slide 82
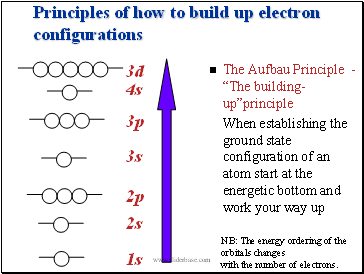
Principles of how to build up electron configurations
The Aufbau Principle - ďThe building-upĒprinciple
When establishing the ground state configuration of an atom start at the energetic bottom and work your way up
2p
3s
3p
3d
1s
2s
NB: The energy ordering of the orbitals changes
with the number of electrons.
4s
Slide 83
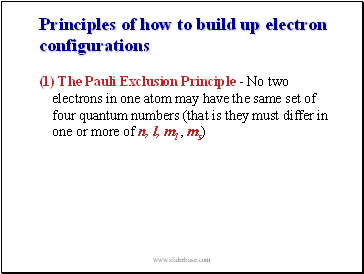
Principles of how to build up electron configurations
(1) The Pauli Exclusion Principle - No two electrons in one atom may have the same set of four quantum numbers (that is they must differ in one or more of n, l, ml , ms)
Slide 84
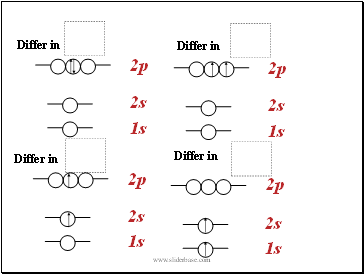
Differ in
Differ in
Differ in
Differ in
Slide 85
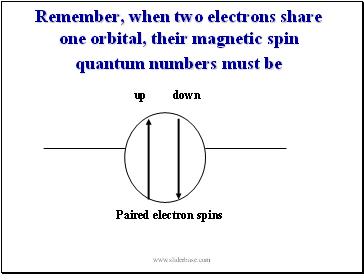
Remember, when two electrons share one orbital, their magnetic spin quantum numbers must be
Slide 86
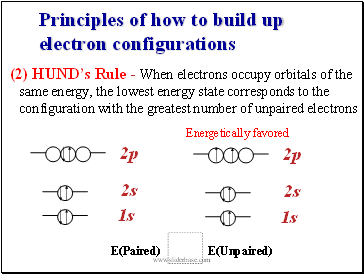
Principles of how to build up electron configurations
(2) HUNDís Rule - When electrons occupy orbitals of the same energy, the lowest energy state corresponds to the configuration with the greatest number of unpaired electrons
E(Paired) E(Unpaired)
Energetically favored
Slide 87
Maximizing the number of parallel spins - The exchange interaction
Quantum mechanical in origin
Arguments based on the fact that total wavefunction has to be with respect to exchange of the electrons (Pauli)
Nothing to do with the fact that electrons are charged!
Result is that each electron pair with parallel spins leads to a lowering of the electronic energy of the atom
Slide 88
Now, letís start remembering
The Pauli Exclusion Principle (only two anitparallel spins in one orbital)
(2) Hundís Rule (parallel spin configuration of lower energy for degenerate orbitals)
(3) Aufbau Principle (from energetic bottom to energetic top)
Slide 89
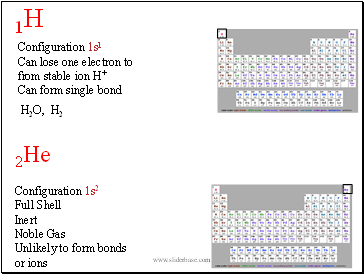
2He
Configuration 1s2
Full Shell
Inert
Noble Gas
Unlikely to form bonds
or ions
1H
Configuration 1s1
Can lose one electron to from stable ion H+
Can form single bond
H2O,
H2
Slide 90
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Resource Acquisition and Transport in Vascular Plants
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Radiation Safety and Operations
- Health Physics
- Sensory and Motor Mechanisms
- Madame Marie Curie
- Heat-Energy on the Move