Atomic Structure and Periodic TrendsPage
9
9
The core electrons will
tend to the outer
electron from the full
nuclear charge.
Slide 72
Shielding and Penetration
If an electron is always outside the core it experiences only a net charge of nucleus and core.
If, however, the electron spends much of its time close to the nucleus (within the core) it will experience a larger nuclear attraction and have a lower energy (more tightly bound).
Hence the energy of the outer electron depends on how much it the core region.
This in turn depends on the type (s, p, d, f etc.) of orbital it is in.
Important
Slide 73
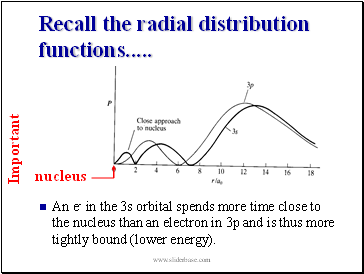
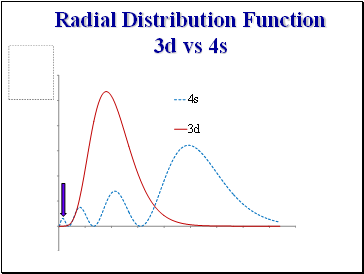
Recall the radial distribution functions .
An e- in the 3s orbital spends more time close to the nucleus than an electron in 3p and is thus more tightly bound (lower energy).
nucleus
Important
Slide 74
Ramifications
The energy of a given quantum state is now no longer simply a function of its principal quantum number but also of its penetration into the core region which depends on the orbital shape (and thus l).
i.e., E=En,l
In general the energies of sub-shells of the same principal quantum number n lie in the order
Important
Slide 75
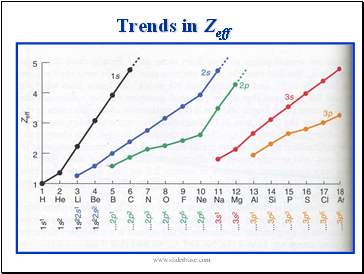
Zeff – the effective nuclear charge
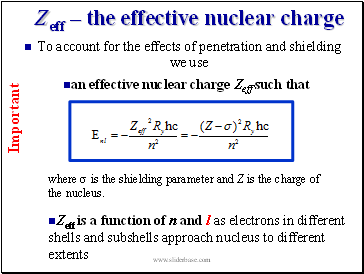
To account for the effects of penetration and shielding we use
Important
an effective nuclear charge Zeff such that
where s is the shielding parameter and Z is the charge of the nucleus.
Zeff is a function of n and l as electrons in different shells and subshells approach nucleus to different extents
Slide 76
Trends in Zeff
Slide 77
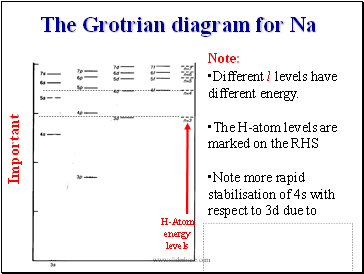
The Grotrian diagram for Na
Note:
Different l levels have different energy.
The H-atom levels are marked on the RHS
Note more rapid stabilisation of 4s with respect to 3d due to
H-Atom
energy
levels
Important
Slide 78
Radial Distribution Function 3d vs 4s
Slide 79
The Aufbau Principle and the Structure of the Periodic Table
Slide 80
Electron Configurations
To obtain a ground state configuration for an atom we apply the Pauli exclusion and the Aufbau principle which states that electrons are added to orbitals in increasing order of energy.
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Direct heat utilization of geothermal energy
- Madame Marie Curie
- Resource Acquisition and Transport in Vascular Plants
- Friction
- Mechanics Lecture
- Radioactivity and Nuclear Reactions
- Sound