Atomic Structure and Periodic TrendsPage
11
11
3Li – Lithium – remember: can’t have s3 (Pauli)
Configuration 1s22s1
Easily ionised to Li+ (1s2)
Alkali Metal
Under standard conditions: lightest metal and least dense element
4Be- Beryllium
Configuration 1s22s2
Be2+ (1s2) stable ion
Alkaline Earth Metal
Toxic: replaces Magnesium from Magnesium activated enzymes due to stronger coordination ability
Slide 91
5B - Boron
Config 1s22s2 2p1
Forms stable covalently bonded molecular networks
Mainly tervalent
Lewis acidity of many of its compounds and multicentre bonding
Chemistry highly diverse and complex
Number of Electrons: 5
Slide 92
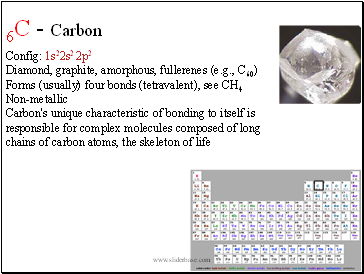
6C - Carbon
Config: 1s22s2 2p2
Diamond, graphite, amorphous, fullerenes (e.g., C60)
Forms (usually) four bonds (tetravalent), see CH4
Non-metallic
Carbon's unique characteristic of bonding to itself is responsible for complex molecules composed of long chains of carbon atoms, the skeleton of life
Slide 93
7N - Nitrogen
Config 1s22s2 2p3
In compounds typically forms three bonds (trivalent), see NH3, N2
Non-metal
N2:
Gaseous, odourless, tasteless
78% of air is N2
Very inert at room temperature (and below) due to strong triple bond
Slide 94
8O
Config 1s22s2 2p4
member of chalcogen group
Normally considered divalent but other oxidations states vary widely
hugely electronegative (see later)
Colourless, odourless, highly reactive gas,
Created biologically from CO2 by green photosynthesizing plants
Paramagnetic (attracted by a magnetic field)
O2:
Slide 95
Exercise 3;
Name the electron configurations of the F and Ne atoms. Which is more stable and why?
Slide 96
9F
Config
Member of the halogen group
Most reactive of all elements
Forms F- readily
highly electronegative!
Does not exist in nature in the elemental state at all because of high reactivity
Slide 97
10Ne - Neon
Config
Full Outer shell
Noble Gas
Unlikely to form bonds or ions
Slide 98
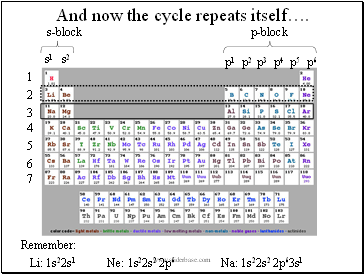
And now the cycle repeats itself….
Remember:
Li: 1s22s1
Na: 1s22s2 2p63s1
Ne: 1s22s2 2p6
1
2
3
4
5
6
7
s1
s2
p1
p2
p3
p4
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Waves & Sound
- Resource Acquisition and Transport in Vascular Plants
- Space Radiation
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Sound
- Newton's laws of motion
- Madame Marie Curie