Atomic Structure and Periodic TrendsPage
17
17
Y(2,1) =y(2)y(1) s(2,1)
exchange
Slide 144
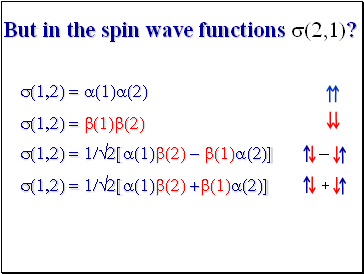
But in the spin wave functions s(2,1)?
s(1,2) = a(1)a(2) s(1,2) = b(1)b(2)
s(1,2) = 1/2[a(1)b(2) - b(1)a(2)] s(1,2) = 1/2[a(1)b(2) +b(1)a(2)]
+
-
Slide 145
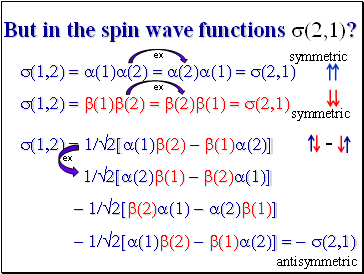
But in the spin wave functions s(2,1)?
s(1,2) = a(1)a(2) = a(2)a(1) = s(2,1) s(1,2) = b(1)b(2) = b(2)b(1) = s(2,1)
s(1,2) = 1/2[a(1)b(2) - b(1)a(2)]
1/2[a(2)b(1) - b(2)a(1)]
- 1/2[b(2)a(1) - a(2)b(1)]
- 1/2[a(1)b(2) - b(1)a(2)] = - s(2,1)
ex
ex
ex
antisymmetric
symmetric
symmetric
Slide 146
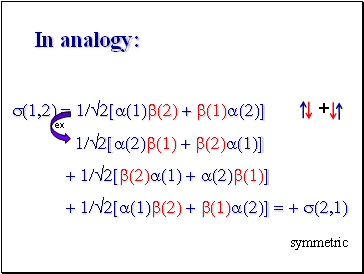
In analogy:
s(1,2) = 1/2[a(1)b(2) + b(1)a(2)]
1/2[a(2)b(1) + b(2)a(1)]
+ 1/2[b(2)a(1) + a(2)b(1)]
+ 1/2[a(1)b(2) + b(1)a(2)] = + s(2,1)
+
ex
symmetric
Slide 147
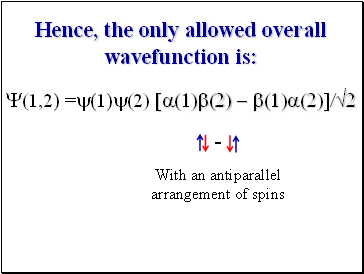
Hence, the only allowed overall wavefunction is:
With an antiparallel arrangement of spins
Y(1,2) =y(1)y(2) [a(1)b(2) - b(1)a(2)]/2
Slide 148
5) Slater’s Rules
Approximate method for estimating the effective nuclear charge
Zeff = Z - S
Where Z is the actual nuclear charge and S is a shielding constant.
Slide 149
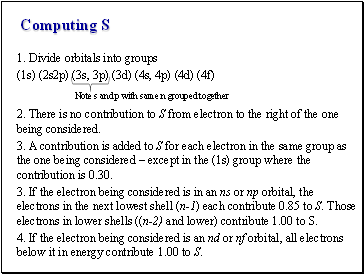
Computing S
1. Divide orbitals into groups
(1s) (2s2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f)
Note s and p with same n grouped together
2. There is no contribution to S from electron to the right of the one being considered.
3. A contribution is added to S for each electron in the same group as the one being considered – except in the (1s) group where the contribution is 0.30.
3. If the electron being considered is in an ns or np orbital, the electrons in the next lowest shell (n-1) each contribute 0.85 to S. Those electrons in lower shells ((n-2) and lower) contribute 1.00 to S.
4. If the electron being considered is an nd or nf orbital, all electrons below it in energy contribute 1.00 to S.
Slide 150
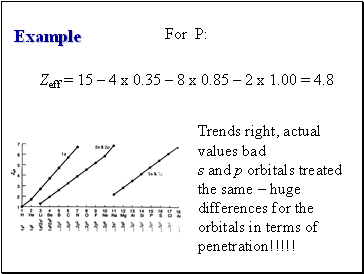
Example
For P:
Zeff = 15 – 4 x 0.35 – 8 x 0.85 – 2 x 1.00 = 4.8
Trends right, actual values bad
s and p orbitals treated the same – huge differences for the orbitals in terms of penetration!!!!!
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Madame Marie Curie
- Motion
- Newton’s Laws of Motion
- Radiation
- Mechanics Lecture
- Ch 9 Nuclear Radiation
- Newton’s Law of Gravity