Atomic Structure and Periodic TrendsPage
6
6
These are the familiar spherical s-orbitals and dumbell-shaped p-orbitals, etc .
Important
Slide 48
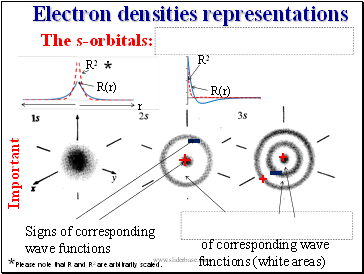
Electron densities representations
Important
+
-
+
+
-
of corresponding wave
functions (white areas)
Signs of corresponding wave functions
R(r)
R2
r
*
*
Please note that R and R2 are arbitrarily scaled.
The s-orbitals:
R2
R(r)
Slide 49
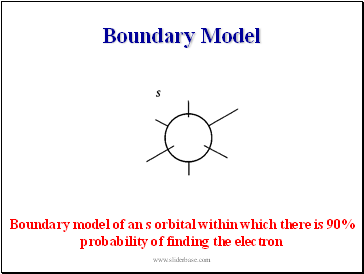
Boundary model of an s orbital within which there is 90% probability of finding the electron
Boundary Model
Slide 50
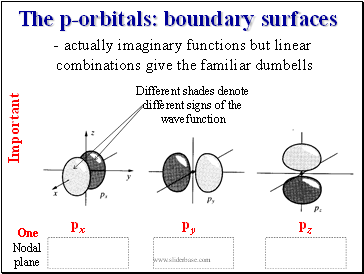
The p-orbitals: boundary surfaces
- actually imaginary functions but linear
combinations give the familiar dumbells
px
pz
py
Important
Different shades denote different signs of the wavefunction
One Nodal plane
Slide 51
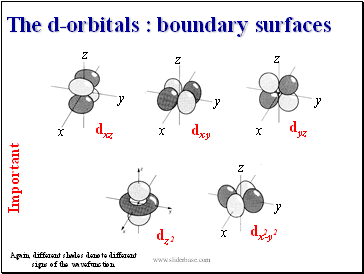
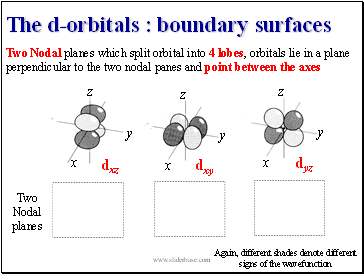
The d-orbitals : boundary surfaces
Important
Again, different shades denote different signs of the wavefunction
dxz
dxy
dyz
dxz
dz
2
dx-y
2
2
z
z
z
z
x
x
x
x
y
y
y
y
Slide 52
The d-orbitals : boundary surfaces
Again, different shades denote different signs of the wavefunction
dxz
dxy
dyz
dzx
Two
Nodal planes
dxz
dxy
dyz
dxz
Two Nodal planes which split orbital into 4 lobes, orbitals lie in a plane perpendicular to the two nodal panes and point between the axes
z
x
y
z
x
y
z
x
y
Slide 53
The d-orbitals : boundary surfaces
2
2
2
z
x
y
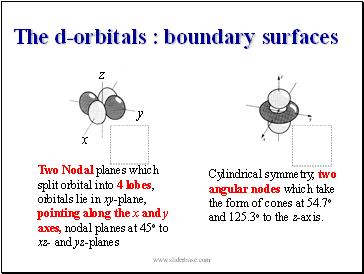
Two Nodal planes which split orbital into 4 lobes, orbitals lie in xy-plane, pointing along the x and y axes, nodal planes at 45o to xz- and yz-planes
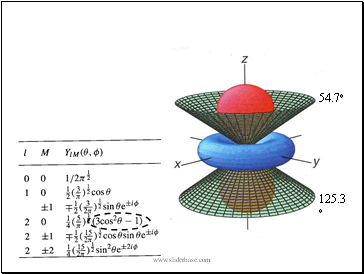
Cylindrical symmetry, two angular nodes which take the form of cones at 54.7o and 125.3o to the z-axis.
Slide 54
54.7o
125.3o
Slide 55
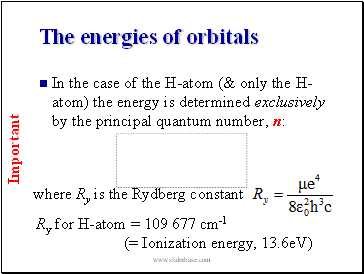
The energies of orbitals
In the case of the H-atom (& only the H-atom) the energy is determined exclusively by the principal quantum number, n:
Important
Slide 56
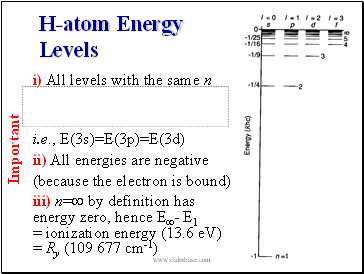
H-atom Energy Levels
i) All levels with the same n
i.e., E(3s)=E(3p)=E(3d)
ii) All energies are negative
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Newton’s Laws of Motion
- Sound
- Gravitation
- Radioactivity and Nuclear Reactions
- The Effects of Radiation on Living Things
- Ch 9 Nuclear Radiation
- Upcoming Classes