Atomic Structure and Periodic TrendsPage
5
5
Slide 38
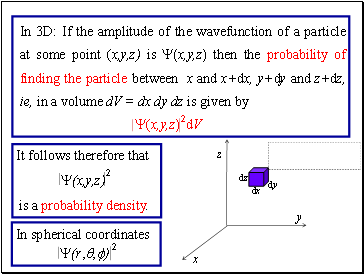
In 3D: If the amplitude of the wavefunction of a particle at some point (x,y,z) is Y(x,y,z) then the probability of finding the particle between x and x+dx, y+dy and z+dz, ie, in a volume dV = dx dy dz is given by
Y(x,y,z) dV
dx
dy
dz
x
z
y
It follows therefore that
2
2
Y(x,y,z)
is a probability density.
In spherical coordinates
2
Y(r,q,f)
Slide 39
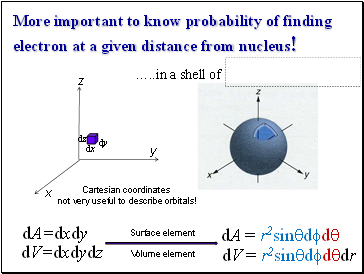
More important to know probability of finding electron at a given distance from nucleus!
dx
dy
dz
x
z
y
dV=dxdydz
Cartesian coordinates
not very useful to describe orbitals!
… in a shell of
dA = r2sinqdfdq
dA=dxdy
dV = r2sinqdfdqdr
Surface element
Volume element
Slide 40
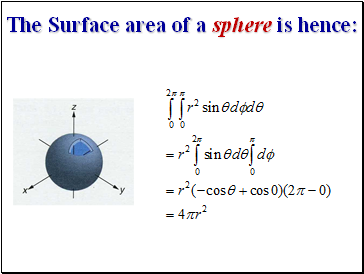
The Surface area of a sphere is hence:
Slide 41
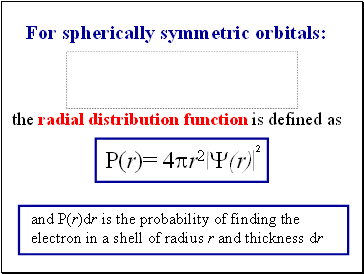
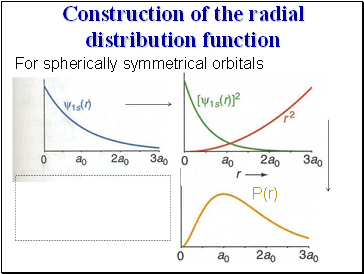
For spherically symmetric orbitals:
the radial distribution function is defined as
2
P(r)= 4pr2 Y(r)
and P(r)dr is the probability of finding the electron in a shell of radius r and thickness dr
Slide 42
Construction of the radial distribution function
For spherically symmetrical orbitals
P(r)
Slide 43
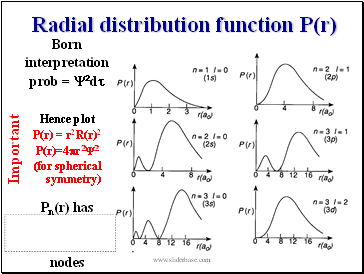
Radial distribution function P(r)
Important
Born
interpretation
prob = Y2dt
Hence plot
P(r) = r2R(r)2
P(r)=4pr2Y2
(for spherical symmetry)
Pn(r) has
nodes
Slide 44
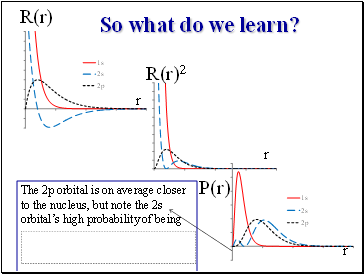
So what do we learn?
R(r)
R(r)2
P(r)
r
r
r
The 2p orbital is on average closer to the nucleus, but note the 2s orbital’s high probability of being
Slide 45
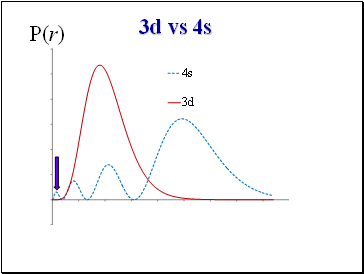
3d vs 4s
P(r)
Slide 46
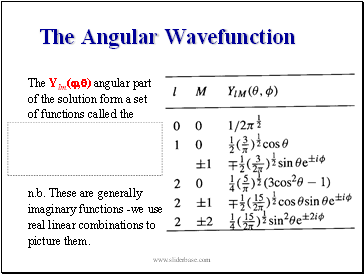
The Angular Wavefunction
The Ylm(,) angular part
of the solution form a set
of functions called the
n.b. These are generally
imaginary functions -we use
real linear combinations to
picture them.
Slide 47
The Shapes of Wavefunctions (Orbitals)
Shapes arise from combination of radial R(r) and angular parts Ylm(,) of the wavefunction.
Usually represented as boundary surfaces which include of the probability density. The electron does not undergo planetary style circular orbits.
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Buoyancy
- Newton’s laws of motion
- Madame Marie Curie
- Heat-Energy on the Move
- Simulation at NASA for the Space Radiation Effort
- Practical Applications of Solar Energy
- Static and Kinetic Friction