Atomic Structure and Periodic TrendsPage
4
4
n = 1, 2, 3, 4, .
l = 0, 1, 2, 3, (n-1)
ml = -l, -l+1, -l+2, 0, ., l-1, l
Important
Slide 29
You are familiar with these .
n is the integer number associated with an orbital
Different l values have different names arising from early spectroscopy
e.g., l =0 is labelled
l =1 is labelled
l =2 is labelled
l =3 is labelled etc .
Important
Slide 30
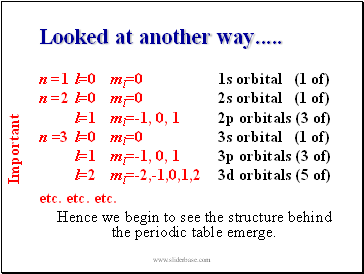
Looked at another way .
n =1 l=0 ml=0 1s orbital (1 of)
n =2 l=0 ml=0 2s orbital (1 of)
l=1 ml=-1, 0, 1 2p orbitals (3 of)
n =3 l=0 ml=0 3s orbital (1 of)
l=1 ml=-1, 0, 1 3p orbitals (3 of)
l=2 ml=-2,-1,0,1,2 3d orbitals (5 of)
etc. etc. etc.
Hence we begin to see the structure behind the periodic table emerge.
Important
Slide 31
Exercise 1;
Work out, and name, all the possible
orbitals with principal
quantum number n=5.
How many orbitals have n=5?
Slide 32
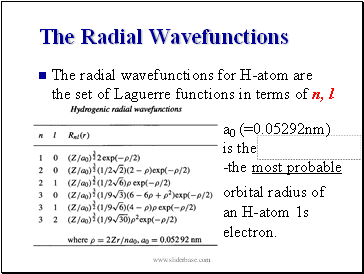
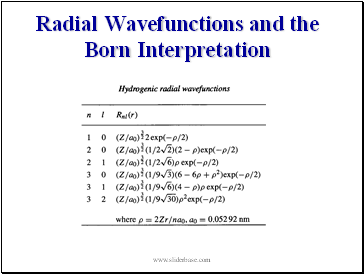
The Radial Wavefunctions
The radial wavefunctions for H-atom are the set of Laguerre functions in terms of n, l
a0 (=0.05292nm)
is the
-the most probable
orbital radius of an H-atom 1s electron.
Slide 33
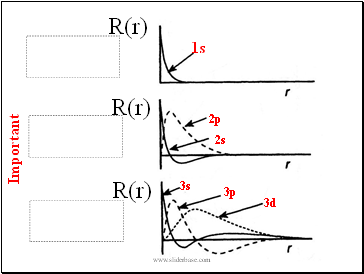
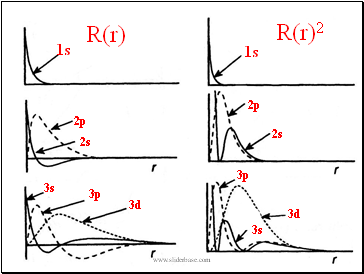
R(r)
1s
2s
2p
3s
3p
3d
Important
R(r)
R(r)
Slide 34
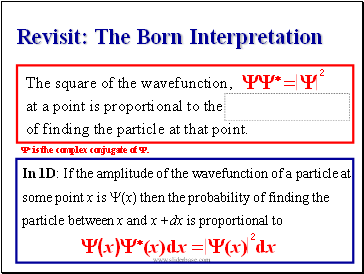
Revisit: The Born Interpretation
The square of the wavefunction,
at a point is proportional to the
of finding the particle at that point.
Y* = Y
In 1D: If the amplitude of the wavefunction of a particle at some point x is Y(x) then the probability of finding the particle between x and x +dx is proportional to
(x)Y*(x)dx = (x)
Y* is the complex conjugate of Y.
2
2
dx
Slide 35
R(r)2
R(r)
1s
2s
2p
3s
3d
1s
2s
2p
3s
3p
3d
3p
Slide 36
Radial Wavefunctions and the Born Interpretation
Slide 37
At long distances from the nucleus, all wavefunctions decay to .
Some wavefunctions are zero at the nucleus (namely, all but the l=0 (s) orbitals). For these orbitals, the electron has a zero probability of being found .
Some orbitals have nodes, ie, the wave function passes through zero; There are such radial nodes for each orbital.
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Solar Energy
- Static and Kinetic Friction
- Newton’s law of universal gravitation
- Sensory and Motor Mechanisms
- Newton's Laws
- Resource Acquisition and Transport in Vascular Plants
- Buoyancy