Atomic Structure and Periodic TrendsPage
14
14
Li(g)
Li+(g) + e-(g)
I1 = 520 kJ/mol
M+(g)
M2+(g) + e-(g)
Li+( g)
Li2+(g) + e-(g)
I2 = 7300 kJ/mol
1st IE
2nd IE
These reactions require energy (endothermic).
Periodic Trends
2) The Ionization Energies (I) within the Periodic Table
I1 = E(M+, g) – E(M, g)
I2 = E(M2+, g) – E(M+, g)
Slide 121
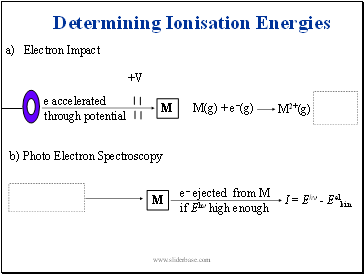
Electron Impact
M
Light (Eh)
M
e- ejected from M
if Eh high enough
I = Eh - Eelkin
+V
Determining Ionisation Energies
e accelerated
through potential
b) Photo Electron Spectroscopy
M(g) + e-(g)
M2+(g) + 2 e-(g)
Slide 122
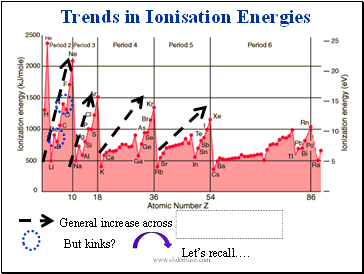
Trends in Ionisation Energies
General increase across periods
But kinks?
Let’s recall….
Slide 123
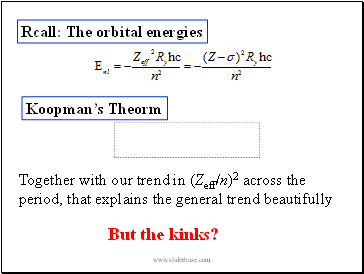
Rcall: The orbital energies
Koopman’s Theorm
I - orbital energy
Together with our trend in (Zeff/n)2 across the period, that explains the general trend beautifully
But the kinks?
Slide 124
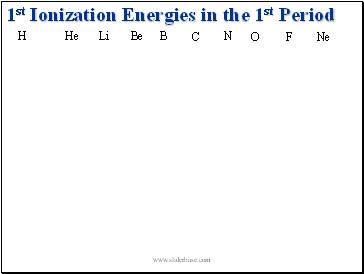
1st Ionization Energies in the 1st Period
H
He
Li
Be
N
O
F
Ne
B
C
Slide 125
Recall: Maximizing the number of parallel spins - The exchange interaction
Quantum mechanical in origin
Arguments based on the fact that total wavefunction has to be antiparallel with respect to exchange of the electrons (Pauli)
Nothing to do with the fact that electrons are charged!
Result is that each electron pair with parallel spins leads to a lowering of the electronic energy of the atom
Slide 126
Space for extra Notes
Slide 127
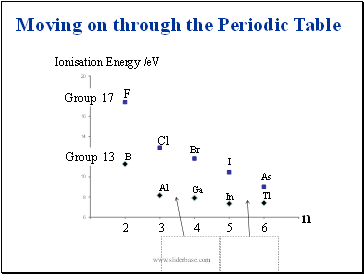
B
Al
Ga
In
Tl
F
Cl
Br
Ionisation Energy /eV
2
3
4
5
6
n
Moving on through the Periodic Table
Group 13
Group 17
I
As
Slide 128
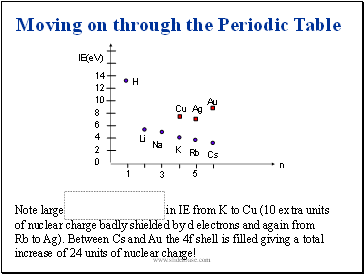
Note large increase in IE from K to Cu (10 extra units of nuclear charge badly shielded by d electrons and again from Rb to Ag). Between Cs and Au the 4f shell is filled giving a total increase of 24 units of nuclear charge!
14
12
10
8
6
4
2
0
IE(eV)
H
Li
Na
K
Rb
Cs
Cu
Ag
Au
Moving on through the Periodic Table
1
3
5
n
Slide 129
More on Ionization Energies
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Mechanics Lecture
- Newton’s third law of motion
- Newton's laws of motion
- Simulation at NASA for the Space Radiation Effort
- Newton’s law of universal gravitation
- Practical Applications of Solar Energy
- Magnetic field uses sound waves to ignite sun's ring of fire