Atomic Structure and Periodic TrendsPage
7
7
(because the electron is bound)
iii) n= by definition has
energy zero, hence E- E1
= ionization energy (13.6 eV)
= Ry (109 677 cm-1)
Important
Slide 57
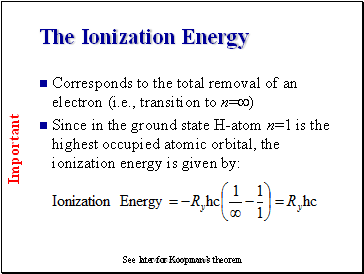
The Ionization Energy
Corresponds to the total removal of an electron (i.e., transition to n=)
Since in the ground state H-atom n=1 is the highest occupied atomic orbital, the ionization energy is given by:
Important
See later for Koopman’s theorem
Slide 58
Other Atoms
Slide 59
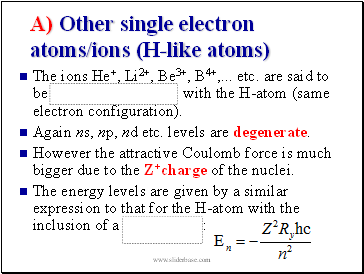
A) Other single electron atoms/ions (H-like atoms)
The ions He+, Li2+, Be3+, B4+, . etc. are said to be with the H-atom (same electron configuration).
Again ns, np, nd etc. levels are degenerate.
However the attractive Coulomb force is much bigger due to the Z+charge of the nuclei.
The energy levels are given by a similar expression to that for the H-atom with the inclusion of a :
Slide 60
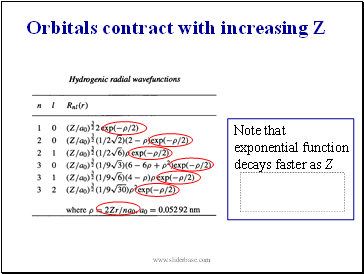
Note that exponential function decays faster as Z
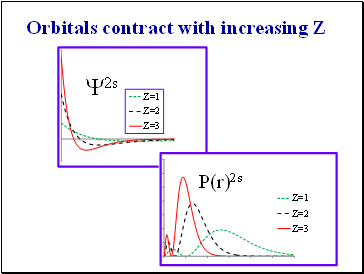
Orbitals contract with increasing Z
Slide 61
Orbitals contract with increasing Z
Y2s
Slide 62
H-like atoms/ions : Summary
The orbitals contract with increasing Z
The effect of the Z2 term is to increase the energy level spacing.
E.g., The energy level spacings in the He+ spectrum are approx. 4 times those in the H-atom whilst in Be3+ they are 16 times larger (Z=4).
This is only approximate because of the slight mass dependence of the Rydberg constant.
Ry(H) = 109 677cm-1 Ry(mass) = 109 737 cm-1
Compare ionisation energies for IE(H) = 13.6eV, IE(He+) = 54.4eV, IE(Li2+)=122.4eV
Slide 63
B) Multi-electron Atoms
Schrödinger equation cannot be solved analytically anymore (apart from He)
Need to develop an approximate picture for multi-electron atoms
2+
-
-
Helium atom
Slide 64
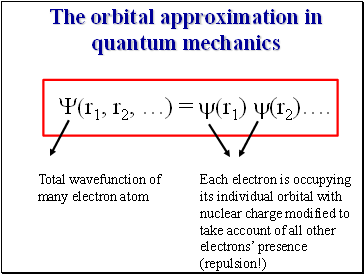
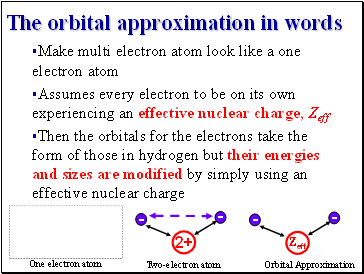
The orbital approximation in quantum mechanics
Y(r1, r2, …) = y(r1) y(r2)….
Total wavefunction of many electron atom
Each electron is occupying its individual orbital with nuclear charge modified to take account of all other electrons’ presence (repulsion!)
Slide 65
The orbital approximation in words
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Resource Acquisition and Transport in Vascular Plants
- Thermal Energy
- Sound
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Buoyancy
- Upcoming Classes
- Radioactivity and Nuclear Reactions