Atomic Structure and Periodic TrendsPage
12
12
p5
p6
s-block
p-block
Slide 99
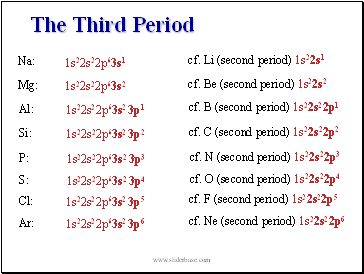
The Third Period
1s22s22p63s1
Na:
cf. Li (second period) 1s22s1
1s22s22p63s2
Mg:
cf. Be (second period) 1s22s2
1s22s22p63s2 3p1
Al:
cf. B (second period) 1s22s22p1
1s22s22p63s2 3p2
Si:
cf. C (second period) 1s22s22p2
1s22s22p63s2 3p3
P:
cf. N (second period) 1s22s22p3
1s22s22p63s2 3p4
S:
cf. O (second period) 1s22s22p4
1s22s22p63s2 3p5
Cl:
cf. F (second period) 1s22s22p5
1s22s22p63s2 3p6
Ar:
cf. Ne (second period) 1s22s22p6
Slide 100
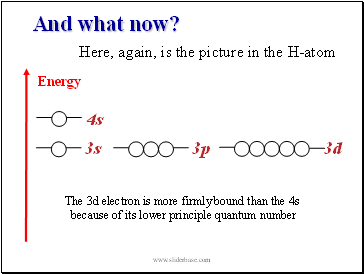
And what now?
3s
3p
3d
4s
Here, again, is the picture in the H-atom
The 3d electron is more firmly bound than the 4s
because of its lower principle quantum number
Energy
Slide 101
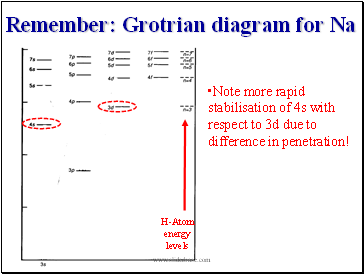
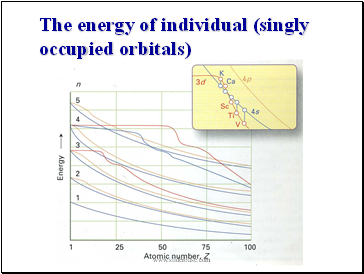
Remember: Grotrian diagram for Na
Note more rapid stabilisation of 4s with respect to 3d due to difference in penetration!
H-Atom
energy
levels
Slide 102
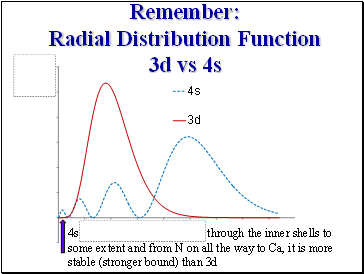
Remember:
Radial Distribution Function 3d vs 4s
4s through the inner shells to some extent and from N on all the way to Ca, it is more stable (stronger bound) than 3d
Slide 103
The energy of individual (singly occupied orbitals)
Slide 104
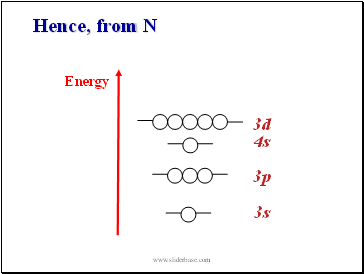
Hence, from N
3s
3p
3d
4s
Energy
Slide 105
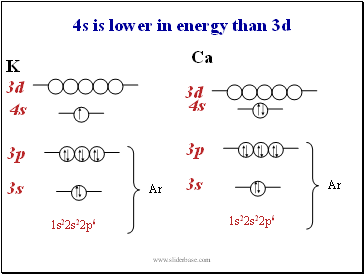
4s is lower in energy than 3d
3s
3p
3d
4s
Ar
1s22s22p6
K
Ca
3s
3p
3d
4s
Ar
1s22s22p6
Slide 106
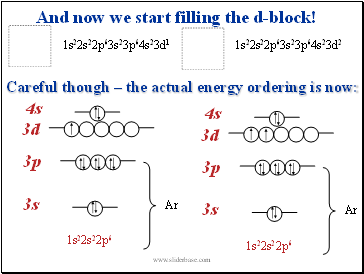
Careful though – the actual energy ordering is now:
3s
3p
3d
4s
Ar
1s22s22p6
3s
3p
3d
4s
Ar
1s22s22p6
1s22s22p63s23p64s23d1
1s22s22p63s23p64s23d2
And now we start filling the d-block!
Slide 107
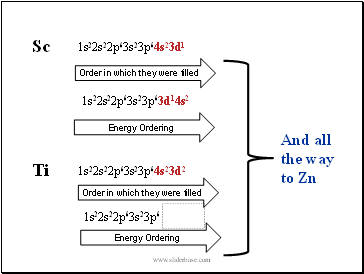
Sc
Ti
1s22s22p63s23p64s23d1
1s22s22p63s23p64s23d2
Order in which they were filled
Energy Ordering
And all the way to Zn
Order in which they were filled
1s22s22p63s23p6
Energy Ordering
1s22s22p63s23p63d14s2
Slide 108
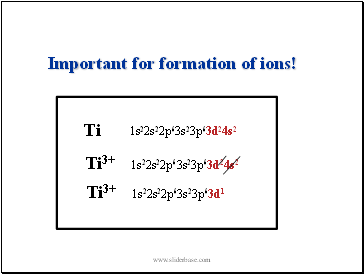
Ti
1s22s22p63s23p63d24s2
Important for formation of ions!
Ti3+
1s22s22p63s23p63d24s2
Contents
- Atomic Structure and Periodic Trends
- Why study atomic electronic structure?
- The Periodic Table
- The Hydrogen Atom
- Energy Levels?
- The Rydberg Formula
- Bohr Theory (old quantum)
- The problem with Bohr Theory
- Quantum mechanical Principles and the Solution of the Schrödinger Equation
- The Results of Quantum Mechanics
- Spherical Polar Coordinates
- The quantum numbers;
- The Radial Wavefunctions
- Revisit: The Born Interpretation
- Radial Wavefunctions and the Born Interpretation
- The Surface area of a sphere is hence:
- Construction of the radial distribution function
- Radial distribution function P(r)
- The Angular Wavefunction
- The Shapes of Wavefunctions (Orbitals)
- Electron densities representations
- The energies of orbitals
- The Ionization Energy
- Other Atoms
- Periodic Trends
- Space for extra Notes
- More on Ionization Energies
- More Periodic Trends
- Appendices
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Thermal Energy
- Health Physics
- Solar Thermal Energy
- Heat-Energy on the Move
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Resource Acquisition and Transport in Vascular Plants